UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_______________________________________________________________
FORM 10-K
_______________________________________________________________
(Mark One)
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For the transition period from July 1, 2020 to December 31 , 2020
Commission file number: 0-26642
_______________________________________________________________
(Exact name of registrant as specified in its charter)
_______________________________________________________________
(State or other jurisdiction
of incorporation or organization)
(Address of principal executive offices)
(I.R.S. Employer
Identification No.)
(Zip Code)
Registrant's telephone number, including area code: (801 ) 584-3600
Securities registered pursuant to Section 12(b) of the Exchange Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||||||||
Securities registered pursuant to Section 12(g) of the Exchange Act: None
_______________________________________________________________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes x No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer”, “accelerated filer”, “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| x | Accelerated filer | ☐ | ||||||||||||
| Non-accelerated filer | ☐ | Smaller reporting company | ||||||||||||
| Emerging growth company | ||||||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Indicate by check mark whether the registrant has filed a report on and attestation to its management's assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the registrant's common stock held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate), computed by reference to the price at which the common stock was last sold on June 30, 2020 was $847,193,483 .
As of March 9, 2021 the registrant had 76,297,150 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
TABLE OF CONTENTS
| Page | ||||||||
2
Cautionary Statement Regarding Forward-Looking Statements
The Securities and Exchange Commission encourages companies to disclose forward-looking information so that investors can better understand a company’s future prospects and make informed investment decisions. This Transition Report on Form 10‑K contains such “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995.
Words such as “may,” “anticipate,” “estimate,” “expects,” “projects,” “intends,” “plans,” “believes,” “seek,” “could,” “continue,” “likely,” “will,” “strategy” and “goal” and words and terms of similar substance used in connection with any discussion of future operating or financial performance, identify forward-looking statements. All forward-looking statements are management’s present expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to:
•uncertainties associated with COVID-19, including its possible effects on our operations and the demand for our products and services;
•risks related to our ability to efficiently and flexibly manage our business amid uncertainties associated with COVID-19;
•the risk that sales and profit margins of our existing molecular diagnostic tests and pharmaceutical and clinical services may decline or that we may not be able to operate our business on a profitable basis;
•risks related to our ability to generate sufficient revenue from our existing product portfolio or in launching and commercializing new tests;
•risks related to changes in governmental or private insurers’ coverage and reimbursement levels for our tests or our ability to obtain reimbursement for our new tests at comparable levels to our existing tests;
•risks related to increased competition and the development of new competing tests and services;
•the risk that we may be unable to develop or achieve commercial success for additional molecular diagnostic tests and pharmaceutical and clinical services in a timely manner, or at all;
•the risk that we may not successfully develop new markets for our molecular diagnostic tests and pharmaceutical and clinical services, including our ability to successfully generate revenue outside the United States;
•the risk that licenses to the technology underlying our molecular diagnostic tests and pharmaceutical and clinical services tests and any future tests are terminated or cannot be maintained on satisfactory terms;
•risks related to delays or other problems with operating our laboratory testing facilities;
•risks related to public concern over genetic testing in general or our tests in particular;
•risks related to regulatory requirements or enforcement in the United States and foreign countries and changes in the structure of the healthcare system or healthcare payment systems;
•risks related to our ability to obtain new corporate collaborations or licenses and acquire new technologies or businesses on satisfactory terms, if at all;
•risks related to our ability to successfully integrate and derive benefits from any technologies or businesses that we license or acquire;
•risks related to our projections about the potential market opportunity for our products;
•the risk that we or our licensors may be unable to protect or that third parties will infringe the proprietary technologies underlying our tests; the risk of patent-infringement claims or challenges to the validity of our patents;
•risks related to changes in intellectual property laws covering our molecular diagnostic tests and pharmaceutical and clinical services, or patents or enforcement, in the United States and foreign countries;
•risks of new, changing and competitive technologies and regulations in the United States and internationally;
•the risk that we may be unable to comply with financial operating covenants under our credit or lending agreements:
•the risk that we will be unable to pay, when due, amounts due under our credit or lending agreements;
•risks related to the material weakness identified in our internal control over financial reporting, including the impact thereof and our remediation plan; and
•other factors discussed under the heading “Risk Factors” contained in Item 1A of this Transition Report.
3
In light of these assumptions, risks and uncertainties, the results and events discussed in the forward-looking statements contained in this Transition Report or in any document incorporated by reference might not occur. Stockholders are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date of this Transition Report. We are not under any obligation, and we expressly disclaim any obligation, to update or alter any forward-looking statements, whether as a result of new information, future events or otherwise. All forward-looking statements in this Transition Report attributable to us or to any person acting on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section.
“We,” “us,” “our,” “Myriad” and the “Company” as used in this Transition Report on Form 10‑K refer to Myriad Genetics, Inc., a Delaware corporation, and its subsidiaries.
“Myriad,” BRACAnalysis, BRACAnalysis CDx, BART, COLARIS, COLARIS AP, MELARIS, myPath, myPlan, myChoice, myRisk, Myriad myRisk, PANEXIA, PREZEON, Prolaris, myChoice CDx, Vectra, Vectraview, TruCulture, DiscoveryMAP, RodentMap, GeneSight, and EndoPredict are registered trademarks or trademarks of Myriad.
4
PART I
Item 1. BUSINESS
Transition Period
On October 9, 2020, our Board of Directors approved a change in our fiscal year end from the last day of June to a calendar fiscal year ending on the last day of December of each year, effective January 1, 2021. In this Transition Report, references to “fiscal year” refer to years ending June 30. References in this report to the “transition period” refer to the six-month period ended December 31, 2020.
Overview
We are a leading precision medicine company acting as a trusted advisor to transform patient lives through molecular diagnostics and are one of the largest specialty molecular diagnostic laboratories in the world. Since our founding in 1992, we have performed tests for approximately five million patients. Through our proprietary technologies, we believe we are positioned to identify important disease genes, the proteins they produce, and the biological pathways in which they are involved to better understand the genetic basis of human disease. We believe that identifying these biomarkers (DNA, RNA and proteins) will enable us to develop novel molecular diagnostic tests that can provide important information to solve unmet medical needs.
Our Mission
As a leader in genetic testing and precision medicine, Myriad Genetics advances the health and well-being of our patients and empowers our patients and healthcare providers with life-changing genetic insights. We apply the power of precision medicine to reveal answers that help better diagnose, treat and prevent disease. Myriad discovers and commercializes genetic tests that determine the risk of developing disease, accurately diagnose disease, assess the risk of disease progression, and guide treatment decisions across medical specialties where our genetic expertise can contribute to better health outcomes and lower costs.
Business Updates
We made the following recent announcements of publications, collaborations and coverage decisions:
•Announced that Myriad recently signed a contract with the majority of the affiliated health plans of Anthem Blue Cross Blue Shield, which returns all Myriad products to in-network status. The new contract will aid in providing easier access to testing for patients and providers.
•Announced a strategic collaboration with Illumina, Inc. to create a kit-based version of the myChoice® companion diagnostic (CDx) test for select international markets.
•Received Japanese reimbursement for the Myriad myChoice® Diagnostic System, which helps determine if women with ovarian cancer will benefit from the PARP inhibitor, Zejula® (niraparib).
•Announced that technological enhancements to Myriad's Foresight® carrier screen test increased the detection rate for alpha thalassemia inherited blood conditions from 90% to >99% in high-risk ethnicities such as Hispanic patients where the risk of alpha thalassemia can be 200 times greater than the risk of cystic fibrosis. These changes reduced the risk of a false negative by 10 times and improved the accuracy of the Foresight test for ethnic minority populations.
•The scientific journal Genetics in Medicine published a study demonstrating that Myriad’s proprietary AMPLIFY® technology increases the accuracy of the Prequel prenatal screen for five common microdeletions by an average of 9 times.
•Received new local coverage determination for the Prolaris® prostate cancer test from two administrative contractors for the Centers for Medicare & Medicaid Services.
5
•Received acceptance for a new study publication in The Prostate demonstrating high accuracy for Prolaris in predicting metastases and disease specific mortality in men following a radical prostatectomy and announced that a new study in Clinical Genitourinary Cancer demonstrated that the Prolaris test can accurately predict which patients will benefit from multi-modality therapy.
•Shared new data at the American College of Rheumatology annual meeting further demonstrating that Vectra® testing and three additional biomarkers, combined with traditional risk factors, can predict the risk of cardiovascular events in patients with rheumatoid arthritis.
•Presented a new study highlighting how riskScore, a proprietary tool used to evaluate a woman’s risk of developing breast cancer, can accurately provide breast cancer risk information in the form of a personalized assessment model for women carrying a pathogenic variant in the ATM gene.
•Announced that the German Federal Joint Committee (G-BA) has successfully completed the method evaluation assessment for the EndoPredict® breast cancer prognostic test. The positive decision means that EndoPredict can be made available to all patients with statutory health insurance in Germany as a benefit of the statutory health insurance scheme.
•Announced a new publication in Psychiatry Research that demonstrated that the GeneSight combinatorial test was superior to single gene testing using the Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines. In a sub-analysis utilizing the GUIDED study data, only the GeneSight combinatorial approach was able to accurately predict variations in outcomes for patients with depression and statistically significantly predicted remission, response, and symptom improvement. Also announced a meta-analysis of 1,556 patients based upon four prospective controlled clinical trials that was published in Pharmacogenomics. The meta-analysis demonstrated statistically significant improvements in remission, response and symptom improvement. We are actively in discussions with commercial payers based upon these positive data sets.
•Announced that we are exploring strategic alternatives for the Myriad RBM, Myriad Dermatology, and Myriad Autoimmune business units.
Our Business Strategy
Personalized genetic data, digital, and virtual consumer trends are converging to change traditional models of care. Significant growth opportunities exist to help patient populations with pressing healthcare needs through innovative solutions and services. We look to capitalize on those trends by focusing on the following strategic priorities:
•Put patients and customers first by improving the experience through new digital tools;
•Build new commercial capabilities;
•Elevate core products to full potential; and
•Create new avenues for growth.
To execute on these priorities, we are first focused on the near term opportunities, including prioritizing product innovation, research and technology initiatives, and defining and deploying a customer-centric technology-enabled commercial model. We believe that by reorganizing around these strategic priorities, we will be able to reduce complexity and cost, improve our financial performance, build a more effective and cost-efficient sales model, and enhance our reimbursement and revenue cycle capabilities. With a foundation of financial, commercial, operational and technological strength, we will accelerate growth as we invest in innovation, research and partnerships, develop capabilities to support our sales team with new digital tools and add more direct-to-consumer engagement, and build commercial capabilities to support new products and potential mergers and acquisitions.
6
Molecular Diagnostic Testing
Our molecular diagnostic tests are designed to analyze genes and their expression levels and corresponding proteins to assess an individual’s risk for developing disease later in life; accurately diagnose disease; determine a patient’s likelihood of either responding to a particular drug or disease recurrence; and assess a patient’s risk of disease progression. Provided with this valuable information, physicians may more effectively manage their patients' healthcare.
myRisk™ Hereditary Cancer: DNA sequencing test for assessing the risks for hereditary cancers. Our myRisk Hereditary Cancer test is designed to determine a patient’s hereditary cancer risk for breast, ovarian, colorectal, endometrial, melanoma, pancreatic, prostate, and gastric cancers. The test analyzes 35 separate genes to look for deleterious mutations that put a patient at a substantially higher risk than the general population for developing one or more of these cancers. All 35 genes in the panel are well documented in clinical literature for the role they play in hereditary cancer and have been shown to have actionable clinical interventions for the patient to facilitate earlier cancer detection, lower disease risk or reduce risk of cancer recurrence. The myRisk Genetic Test Result and myRisk Management Tool® summarize medical society guidelines for managing a patient with a genetic mutation in view of their personal and family history of cancer. myRisk Hereditary Cancer testing identifies more mutation carriers than our BRACAnalysis® and COLARIS® tests combined.
BRACAnalysis CDx®: DNA sequencing test to help determine the appropriate therapy for patients with metastatic breast, ovarian, metastatic pancreatic, or metastatic prostate cancer with deleterious or suspected deleterious germline BRCA variants. Results of the test are used as an aid to identify patents who are eligible for treatment with U.S. Food and Drug Administration approved PARP inhibitors. This is an in vitro diagnostic device intended for the qualitative detection and classification of variants in the protein coding regions and intron/exon boundaries of the BRCA1 and BRCA2 genes using genomic DNA obtained from whole blood specimens collected in EDTA.
MyChoice® CDx: the most comprehensive tumor test that determines homologous recombination (HRD) status in patients with ovarian cancer. This FDA-approved test helps provide information on the magnitude of benefit for PARP inhibitor therapy. HRD status is determined using two independent methods: BRCA1 and BRCA2 status that encompasses sequence variants and large rearrangements, and Genomic Instability Status (GIS) encompassing loss of heterozygosity, telomeric allelic imbalance and large-scale state transitions across the entire genome. The combination of these methods is a more comprehensive way to measure HRD status, versus either one alone.
Prolaris®: RNA expression tumor analysis for assessing the aggressiveness of prostate cancer. Our Prolaris test is a gene expression assay that assesses whether a patient is likely to have a slow growing, indolent form of prostate cancer that can be safely monitored through active surveillance, or a more aggressive form of the disease that may warrant aggressive intervention such as a radical prostatectomy or radiation therapy. The Prolaris test was developed to improve physicians’ ability to predict disease outcome and thereby to optimize patient treatment.
EndoPredict®: RNA expression test for assessing the aggressiveness of breast cancer. The EndoPredict test is a next-generation RNA expression test used to determine which women with breast cancer may benefit from chemotherapy. EndoPredict predicts the likelihood of metastases to help guide treatment decisions for chemotherapy and extended endocrine therapy. EndoPredict has been shown to accurately predict risk of distant recurrence in Her 2-, ER+, node negative and node positive breast cancer patients with no confusing intermediate results in 13 published clinical studies with more than 2,200 patients and is CE marked.
Foresight®: a prenatal test for future parents to assess their risk of passing on a recessive genetic condition to their offspring. The test screens for "carrier status" of up to 175 serious and clinically actionable conditions. The test has been shown to have a detection rate of 99% across all ethnicities. Studies have shown that with prior knowledge of recessive genetic conditions, 76% of patients took preventive actions such as in-vitro fertilization with pre-implantation genetic testing to reduce the risk of having an affected offspring.
Prequel®: a non-invasive prenatal screening test conducted using maternal blood to screen for severe chromosomal disorders in a fetus. The test uses whole genome sequencing to test for trisomies and monosomies in all 23 chromosomal pairs including the sex chromosomes, along with microdeletions associated with common genetic diseases. Prequel has a low test failure rate at less than 1 in 1,000 patients and has been validated in multiple clinical studies to be highly accurate.
7
GeneSight®: DNA genotyping test to aid psychotropic drug selection for patients suffering from depression, anxiety, and other psychiatric conditions. GeneSight® is for use by health-care professionals seeking patient-specific information on gene-drug interactions when contemplating an alteration in neuropsychiatric medication for patients diagnosed with major depressive disorder (MDD) who are suffering with refractory moderate to very severe depression after at least one prior neuropsychiatric medication failure. Because genes influence the way a person’s body responds to specific medications, the medications may work differently for each person. Using DNA gathered with a simple cheek swab, GeneSight analyzes a patient’s genes and provides individualized information to help healthcare providers select medications that better match the patient’s genes. Multiple clinical studies have shown that when clinicians used GeneSight to help guide treatment decisions, patients were more likely to respond compared to standard of care.
Sales and Marketing
We sell our tests through our own direct sales force and marketing efforts in the United States, France, Germany and Japan, and service additional global accounts through indirect sales channels. Our United States sales force is comprised of approximately 900 individuals across five separate sales channels. We continue to evaluate our sales and marketing channels including increased digital marketing, direct to patient marketing, enhanced virtual sales tools, and inside sales teams to drive efficiency in our model. We intend to elevate our existing products to their full potential by increasing awareness, access, and differentiation of our products, while we reinforce clinical utility data, and maximize cross-selling and synergies across our portfolio.
Research and Development
We plan to continue to use our proprietary DNA sequencing, RNA expression and protein analysis technologies, including our supporting bioinformatics and robotic technologies, in an effort to efficiently discover important biomarkers and to understand their role in human disease. Based on these biomarkers, we plan to develop highly accurate, informative tests that may help physicians better manage their patients’ healthcare. We believe that our technologies provide us with a significant competitive advantage and the potential for numerous product opportunities. For the transition period ended December 31, 2020, and fiscal years ended June 30, 2020, 2019 and 2018, we incurred research and development expense of $35.8 million, $77.2 million, $85.9 million and $70.8 million, respectively.
Industry and Competition
Patients, providers, payors and health systems are looking to apply the power of molecular diagnostics and precision medicine to achieve improved clinical outcomes and lower cost. Key industry trends include:
•Accelerating shifts in consumer engagement, early detection, home-based care models, telemedicine and virtual care;
•Disruption in the way outpatient care is delivered in the wake of the COVID-19 pandemic, coupled with broadened awareness of the vital role of diagnostic testing;
•Expanding access to genetic insights, particularly among underserved populations with increased focus on disparities in healthcare outcomes and access for challenged communities; and
•Growth in personalized medicine and the interest in new partnership models to advance companion diagnostics and serve patients with specific treatments based on their own genetic makeup and biology.
These market trends create new opportunities to position Myriad Genetics, and our products and services, for growth and commercial success through enhanced customer service levels and a stronger alignment of our value proposition with physicians and payors. Our record of innovation will be leveraged not only in research and development and technology, but also in go-to-market approaches and other applications so we can adapt quickly to customer preferences and market dynamics.
Oncology
In oncology, we offer genetic testing for patients who have cancer and companion diagnostic tests that work with corresponding drugs. Our major competitors in the oncology market include Invitae, Ambry Genetics, Quest Diagnostics, LabCorp, Exact Sciences, Foundation Medicine, and other commercial and academic laboratories.
8
We believe the key opportunities and potential catalysts are our international expansion of companion diagnostics and market expansion through new clinical guidelines. We recently signed an agreement with Illumina to offer a myChoice CDx kit as part of their Trusight oncology offering. This kit will be targeted to international markets and will increase global access to our myChoice CDx solution. The agreement combines Illumina's expertise in next-generation sequencing with Myriad's proprietary myChoice CDx assay technology to advance comprehensive genetic profiling of tumor samples and drive improved outcomes in oncology. This collaboration reflects our focus on partnering with high-caliber healthcare leaders to bring innovative solutions to the oncology market.
We currently offer our FDA-approved BRACAnalysis CDx test as a companion diagnostic for the prediction of response to a class of drugs called PARP inhibitors. Currently, we are the only laboratory with an FDA-approved germline test for this indication and have received approvals from the FDA in ovarian cancer, metastatic breast cancer, pancreatic cancer, and advanced prostate cancer.
In May 2020, we received FDA approval for the myChoice CDx® test for use as a companion diagnostic by healthcare professionals to identify advanced ovarian cancer patients with positive homologous recombination deficiency status, who are eligible or may become eligible, for treatment with Lynparza (olaparib) in combination with bevacizumab. We also have started to receive myChoice CDx samples from Japan following regulatory approval of myChoice CDX to be used as a companion diagnostic for olaparib in fall 2020. With BRACAnalysis CDx, we also received regulatory approval in Japan for new indications in pancreatic and prostate cancer.
Women’s Health
In the women’s health market, we serve women assessing their genetic predisposition to cancer, offer prenatal tests for the assessment of fetal chromosomal disorders, and screen prospective parents for recessive genetic conditions that can be passed on to their offspring. We compete with multiple companies including large national reference laboratories, specialty laboratories, academic/university laboratories, and kit-based products with our myRisk, riskScore, Foresight, and Prequel tests. Some of our major competitors include Invitae, Natera, Ambry Genetics, Quest Diagnostics, LabCorp, Progenity, and Sema4. We compete mainly based on our test breadth and accuracy, commercial scale in the prenatal market, and the quality of our customer service and informatics tools.
We see opportunities to improve our economics and improve the customer experience on these products. We are focused on the reimbursement for carrier screening and finding streamlined patient payment models. We are also developing new global riskScore capabilities to expand access to genetic insights for more ethnic groups and providing new channels for customers to access our products. These include a new online myRisk portal to engage with patients and physicians, patient cost estimators across our product lines, and AI-based tools for interacting with patients.
The launch of our new AMPLIFY™ technology in 2020 further increases the accuracy of the Prequel test, enabling more women to receive highly accurate test results and avoid invasive procedures regardless of body mass index (BMI), race, or ethnicity.
Mental Health
In mental health, we help physicians understand how genetic alterations impact patient response to antidepressants and other drugs. Our GeneSight Psychotropic test meets a significant unmet clinical need and is the leading product to help physicians anticipate patient response to psychotropic drugs, the selection of which has historically been done through trial and error based approaches. The test is clinically proven to improve response rates in patients. Our major competitors in this market include Genomind, AltheaDx, and numerous other commercial and academic laboratories.
Key drivers for this market include primary care expansion, commercial payer reimbursement, and telehealth partnerships. We are broadening access to GeneSight among front-line providers of mental health treatment, including primary care physicians and nurse practitioners who treat the majority of depression and anxiety patients, through the expansion of sales and digital marketing capabilities.
Across the core specialties of our business – women’s health, oncology, and mental health – we have numerous opportunities to elevate our products. We have a respected portfolio and internationally recognized scientific know-how. Our long-standing record of innovation will be leveraged not only in research and development and technology, but also in go-to-market strategies and other applications so we can advance our mission of improving lives while adapting quickly to customer preferences and market dynamics.
9
Seasonality
We have historically experienced seasonality in our testing business. The quarter ending December 31 is generally strong as we see an increase in volumes from patients who have met their annual insurance deductible. Conversely, in the quarter ending March 31 we see a decrease in volumes due to the annual reset of patient deductibles. Additionally, the volume of testing is negatively impacted by the summer season, which is generally reflected in the quarter ended June 30. Due to the global pandemic, we cannot predict if seasonality will follow the same pattern as in prior years.
Human Capital Management
Myriad is dedicated to being a trusted advisor to transform patient lives through pioneering molecular diagnostics. We believe the success of our business will depend, in part, on our ability to attract and retain qualified personnel. To ensure that Myriad can attract, retain and motivate the exceptional people needed to carry out our mission, we maintain competitive compensation and benefits programs designed to help our employees balance their work and personal lives.
As of December 31, 2020, we have approximately 2,700 full-time equivalent employees. Most of our employees are engaged directly in research, development, production, sales and marketing activities. Our employees are not covered by a collective bargaining agreement, and we consider our relations with our employees to be good.
Oversight and Management. We regularly conduct surveys to seek feedback from our employees on a variety of topics, including but not limited to, employee engagement, strengths, focus areas and culture drivers. The results are reviewed by the Board and senior leadership, who analyze areas of progress or deterioration and prioritize actions and activities in response to this feedback to drive meaningful improvements in employee engagement.
Compensation and Benefits Program. Our compensation program is designed to attract and reward talented individuals who possess the skills necessary to support our business objectives, assist in the achievement of our strategic goals and create long-term value for our stockholders. We provide employees with competitive cash compensation programs and stock ownership opportunities. In addition to cash and equity compensation, we also offer benefits such as medical, dental and vision healthcare coverage, insurance and disability coverage, 401(k) investment plans with company matching, tax advantaged savings accounts, paid time off and leaves of absence, employee assistance programs, community outreach programs, training and development opportunities and wellness programs.
As a united team, these values have allowed us to rise to the occasion of this pandemic, together. During the pandemic, we have aligned with CDC guidelines to protect our employees, and in addition to all employees wearing masks and adhering to social distancing guidelines, we also perform contact tracing when an exposure occurs. We recently began offering COVID-19 test kits to employees who may have come into contact with someone exposed to COVID-19 as an additional benefit to our employees and in order to limit disruption to our business operations. There is power in caring, team, diversity, equality and belonging.
Career Development and Training. We offer several career development and training opportunities to our employees, including an extensive curriculum of company-sponsored technical, business and leadership courses, on-the-job training and a support network to all new employees, tuition reimbursement for approved external training and educational pursuits.
Diversity and Inclusion. We believe in equality and in the power of diversity and inclusion. We are striving to make Myriad a place where all employees have a sense of belonging. We recently implemented a diversity, equity and inclusion council that is committed to ensuring that everyone at Myriad has equal opportunities to participate in programs aimed to foster diversity and inclusion. Our focus for diversity is on seeking diverse candidate slates and interview panels for select roles at the leadership level, which is where we have the greatest opportunity to expand. Our focus for inclusion is on embedding inclusion practices into key programs.
Myriad also sponsors the Women’s Leadership Forum, which is a Company initiative that mentors, fosters, encourages and inspires women in all stages of their career by providing access to role models, peer groups, and other valuable resources to help women pursue their career ambitions.
10
Patents and Proprietary Rights
We own or have license rights to various issued patents and patent applications in the United States and foreign countries. These patents and patent applications relate to a variety of subject matter, including diagnostic biomarkers, gene expression signatures, assays, assay reagents, informatics and data analytics, methods for determining genetic predisposition, methods for disease diagnosis, methods for determining disease progression, methods for determining disease treatment, and general molecular diagnostic techniques. For some of the patent assets, we hold rights through exclusive or non-exclusive license agreements. Material patent assets relating to our tests that generate material revenue are described in the following table:
| Test | Patent Assets | Expiration | Claims | ||||||||
| Vectra | We own or hold an exclusive license to one or more issued U.S. patents and pending patent applications in the U.S. and other jurisdictions relating to Vectra® testing. | The issued U.S. patent has a term expected to expire in 2031 and these U.S. applications, if issued as patents and depending on term adjustments or terminal disclaimers if applicable, are expected to have similar expiration timeframes. | These patents and applications contain multiple claims including but not limited to claims relating to biomarkers, kits, systems and methods for measuring and monitoring inflammatory disease activity. | ||||||||
| Prolaris | We own or hold an exclusive license to one or more issued patents and pending patent applications in the U.S. and other jurisdictions relating to Prolaris® testing. | These issued U.S. patents have terms expected to begin expiring in 2032 and these applications, if issued as patents and depending on term adjustments or terminal disclaimers if applicable, are expected to have similar expiration timeframes. | These patents and applications contain multiple claims including but not limited to claims relating to biomarkers, kits, systems and methods for detecting, diagnosing, prognosing and selecting therapy for prostate cancer. | ||||||||
| EndoPredict | We own or hold an exclusive license to one or more issued patents and pending patent applications in the U.S., Europe and other jurisdictions relating to EndoPredict® testing. | These issued patents have terms expected to begin expiring in 2031 and these applications, if issued as patents and depending on term adjustments or terminal disclaimers if applicable, are expected to have similar expiration timeframes. | These patents and applications contain multiple claims including but not limited to claims relating to biomarkers, kits, systems and methods for prognosing and selecting therapy for breast cancer. | ||||||||
| myChoice CDx | We own or hold an exclusive license to one or more issued patents and pending patent applications in the U.S. and other jurisdictions relating to myChoice® CDx testing. | These issued patents have terms expected to expire in 2032 and these applications, if issued as patents and depending on term adjustments or terminal disclaimers if applicable, are expected to have similar expiration timeframes. | These patents contain multiple claims including but not limited to claims relating to biomarkers, kits, systems and methods for detecting homologous recombination deficiency and selecting therapy based on such detection. | ||||||||
| GeneSight | We own or hold an exclusive license to one or more issued patents and pending patent applications in the U.S. and other jurisdictions relating to GeneSight® testing. | These issued patents have terms expected to begin expiring in 2024 and these applications, if issued as patents and depending on term adjustments or terminal disclaimers if applicable, are expected to have similar expiration timeframes. | These patents contain multiple claims including but not limited to claims relating to biomarkers, kits, systems and methods for detecting single nucleotide polymorphisms and selecting and/or optimizing therapy based on such detection. | ||||||||
| Foresight | We own or hold an exclusive license to one or more issued patents and pending patent applications in the U.S. and other jurisdictions that relate to laboratory and informatic methods used to enhance Foresight® testing. | These issued patents have terms expected to begin expiring in 2032 and these applications, if issued as patents and depending on term adjustments or terminal disclaimers if applicable, are expected to have similar expiration timeframes. | These patents contain multiple claims including but not limited to claims relating to systems and methods for detecting genetic sequences. | ||||||||
| Prequel | We own or hold a license to one or more issued patents and pending patent applications in the U.S. and other jurisdictions that relate to laboratory and informatic methods used to enhance Prequel™ testing. | These issued patents have terms expected to begin expiring in 2022 and these applications, if issued as patents and depending on term adjustments or terminal disclaimers if applicable, are expected to have similar expiration timeframes. | These patents contain multiple claims including but not limited to claims relating to systems and methods for detecting genetic sequences. | ||||||||
11
We intend to seek patent protection in the United States and major foreign jurisdictions for these and other inventions which we believe are patentable and where we believe our interests would be best served by seeking patent protection. However, any patents issued to us or our licensors may not afford meaningful protection for our products or technology or may be subsequently circumvented, invalidated or narrowed or found unenforceable. Any patent applications which we have filed, or will file, or to which we have licensed or will license rights may not issue, and patents that do issue may not contain commercially valuable claims. In addition, others may obtain patents having claims which cover aspects of our tests or processes which are necessary for or useful to the development, use or performance of our diagnostic products. Should any other group obtain patent protection with respect to our discoveries, our commercialization of our molecular diagnostic tests could be limited or prohibited.
Others may offer clinical diagnostic genomic laboratory testing services which may infringe patents we control. We may seek to negotiate a license to use our patent rights or decide to seek enforcement of our patent rights through litigation. Patent litigation is expensive, the outcome is often uncertain and we may not be able to enforce our patent rights against others.
Our tests and processes may also conflict with patents which have been or may be granted to competitors, academic institutions or others. In addition, third parties could bring legal actions against us seeking to invalidate our owned or licensed patents, claiming damages, or seeking to enjoin clinical testing, development and marketing of our tests or processes. If any of these actions are successful, in addition to any potential liability for damages, we could lose patent coverage for our tests, be required to cease the infringing activity or obtain a license in order to continue to develop or market the relevant test or process. We may not prevail in any such action, and any license required under any such patent may not be made available on acceptable terms, if at all. Our failure to maintain patent protection for our tests and processes or to obtain a license to any technology that we may require to commercialize our tests and technologies could have a material adverse effect on our business.
We also rely upon unpatented proprietary technology, and in the future may determine in some cases that our interests would be better served by protecting certain technologies as trade secrets or through confidentiality agreements rather than patents or licenses. These include some of our genomic, proteomic, RNA expression, mutation analysis, robotic and bioinformatic technologies which may be used in discovering and characterizing new biomarkers and ultimately used in the development or analysis of molecular diagnostic tests. We also maintain a database of gene mutations and their status as either harmful or benign for some of our tests. To further protect our trade secrets and other proprietary information, we require that our employees and consultants enter into confidentiality and invention assignment agreements. However, those confidentiality and invention assignment agreements may not provide us with adequate protection. We may not be able to protect our rights to such unpatented proprietary technology and others may independently develop substantially equivalent technologies. If we are unable to obtain strong proprietary rights to our processes or tests, competitors may be able to market competing processes and tests.
12
License Agreements
We are a party to license agreements which give us the rights to use certain technologies in the research, development, testing processes, and commercialization of our molecular diagnostic tests and pharmaceutical and clinical services. We may not be able to continue to license these technologies on commercially reasonable terms, if at all. Additionally, patents underlying our license agreements may not afford meaningful protection for our technology or tests or may be subsequently circumvented, invalidated or narrowed, or found unenforceable. Our failure to maintain rights to this technology could have a material adverse effect on our business. We have licenses with the following entities:
| Entity | Subject | Royalties | Expiration | Termination | ||||||||||
| Mayo Foundation for Medical Education and Research (“Mayo”) | Exclusive world-wide license to certain rights of Mayo in intellectual property relating to our GeneSight testing. | We pay Mayo a royalty based on net sales of our GeneSight test. | License expires upon expiration of the last to expire patent right covered by the Mayo agreement, which presently is not anticipated to expire until 2024. | Mayo has the right to terminate the agreement for the uncured breach of any material term of the agreement. | ||||||||||
| Oklahoma Medical Research Foundation (“OMRF”) | Exclusive world-wide right to certain intellectual property rights of OMRF in intellectual property relating to our Vectra testing. | We pay OMRF a royalty based on net sales of our Vectra test. | License agreement ends on expiration of the last to expire patent right covered by the license agreement, which presently is not anticipated to expire until 2031. | OMRF has the right to terminate the license agreement for the uncured breach of any material term of the license agreement. | ||||||||||
| University of Texas M.D. Anderson Cancer Center (“UTMDACC”) | Exclusive world-wide right to certain rights of UTMDACC in intellectual property relating to our myChoice® HRD testing. | We will pay UTMDACC a royalty based on net sales of our myChoice® HRD test. | License agreement ends on expiration of the last to expire patent right covered by the license agreement, which presently is not anticipated to expire until 2032. | UTMDACC has the right to terminate the license agreement for the uncured breach of any material term of the license agreement. | ||||||||||
| Children’s Medical Center in Boston (“CMCC”) | Exclusive world-wide right to certain rights of CMCC in intellectual property relating to our myChoice® HRD testing. | We expect to pay CMCC a royalty based on net sales of our myChoice® HRD test. | License agreement ends on expiration of the last to expire patent right covered by the license agreement, which presently is not anticipated to expire until 2032. | CMCC has the right to terminate the license agreement for the uncured breach of any material term of the license agreement. | ||||||||||
| Institut Curie and INSERM (“INSERM”) | Exclusive world-wide right to certain rights of INSERM in intellectual property relating to our myChoice® HRD testing. | We expect to pay INSERM a royalty based on net sales of our myChoice® HRD test. | License agreement ends on expiration of the last to expire patent right covered by the license agreement, which presently is not anticipated to expire until 2032. | INSERM has the right to terminate the license agreement for the uncured breach of any material term of the license agreement. | ||||||||||
| Illumina, Inc. (“Illumina”) | Non-exclusive license to certain rights held by or licensed to Illumina to intellectual property relating to non-invasive prenatal screening and the Prequel test. | We pay Illumina a royalty based on the volume of Prequel testing administered by us. | License runs for the term of the Illumina agreement and, in any event, expires upon expiration of the last to expire patent right covered by the Illumina agreement. | Illumina has the right to terminate the agreement for the uncured breach of any material term of the agreement. | ||||||||||
Governmental Regulation
Our operations are regulated by federal, state and foreign governmental authorities. Failure to comply with the applicable laws and regulations can subject us to repayment of amounts previously paid to us, significant civil and criminal penalties, loss of licensure, certification, or accreditation, or exclusion from state and federal health care programs. The significant areas of regulation are summarized below.
13
Clinical Laboratory Improvement Amendments of 1988 and State Regulation
Each of our clinical laboratories must hold certain federal, state and local licenses, certifications, and permits to conduct our business. Laboratories in the United States that perform testing on human specimens for the purpose of providing information for the diagnosis, prevention, or treatment of disease are subject to the Clinical Laboratory Improvement Amendments of 1988 (“CLIA”). CLIA requires such laboratories to be certified by the federal government and mandates compliance with various operational, personnel, facilities administration, quality and proficiency testing requirements intended to ensure that testing services are accurate, reliable and timely. CLIA certification also is a prerequisite to be eligible to bill state and federal health care programs, as well as many private insurers, for laboratory testing services. Our laboratories in Salt Lake City, Utah; Austin, Texas; Mason, Ohio; and South San Francisco, California are CLIA certified to perform high complexity tests.
In addition, CLIA requires each of our certified laboratories to enroll in an approved proficiency testing program if performing testing in any category for which proficiency testing is required. Each of our laboratories periodically tests specimens received from an outside proficiency testing organization and then submits the results back to that organization for evaluation. If one of our laboratories fails to achieve a passing score on a proficiency test, then it may lose its right to perform testing. Further, failure to comply with other proficiency testing regulations, such as the prohibition on referral of a proficiency testing specimen to another laboratory for analysis, can result in revocation of the laboratory’s CLIA certification.
As a condition of CLIA certification, each of our laboratories is subject to survey and inspection every other year, in addition to being subject to additional random inspections. The biennial survey is conducted by the Centers for Medicare & Medicaid Services (“CMS”), a CMS agent (typically a state agency), or a CMS-approved accreditation organization. Because our laboratories are accredited by the College of American Pathologists (“CAP”), which is a CMS-approved accreditation organization, they are typically subject to CAP inspections.
Our laboratories are licensed by the appropriate state agencies in the states in which they operate, if such licensure is required. In addition, our laboratories hold state licenses or permits, as applicable, from various states, including, but not limited to, California, New York, Pennsylvania, Rhode Island and Maryland, to the extent that they accept specimens from one or more of these states, each of which requires out-of-state laboratories to obtain licensure.
If a laboratory is out of compliance with state laws or regulations governing licensed laboratories or with CLIA, penalties may include suspension, limitation or revocation of the license or CLIA certificate, assessment of financial penalties or fines, or imprisonment. Loss of a laboratory’s CLIA certificate or state license may also result in the inability to receive payments from state and federal health care programs as well as private third party payors. We believe that we are in material compliance with CLIA and all applicable licensing laws and regulations.
Food and Drug Administration
In the United States, in vitro diagnostic (“IVD”) products are subject to regulation by the FDA as medical devices to the extent that they are intended for use in the diagnosis, treatment, mitigation or prevention of disease or other conditions. They are subject to premarket review and post-market controls that will differ depending on how the FDA classifies a specific IVD. For certain types of tests known as laboratory developed tests (“LDTs”)—which are in vitro diagnostic tests that are designed, manufactured and used within a single laboratory—FDA regulation is less clear than for IVDs. Historically FDA has exercised enforcement discretion for LDTs, which means that FDA generally has not enforced premarket review and other applicable FDA requirements. However, as LDTs have increased in complexity, the FDA has taken a risk-based approach to their regulation. Congress has also signaled interest in clarifying the regulatory landscape for LDTs. In 2020, the Verifying Accurate, Leading-edge IVCT Development (“VALID”) Act was introduced in both chambers of Congress. If enacted, clinical laboratories that develop and offer LDTs and traditional IVD medical device manufacturers would be subjected to the same regulatory oversight. The VALID Act defines both LDTs and IVDs as in vitro clinical tests (“IVCT”) and would establish a new regulatory framework under the Food, Drug and Cosmetic Act (“FDCA”) for the review and oversight of IVCTs. The proposed regulatory framework adopts various concepts from the FDCA, utilizing a risk-based approach that aims to ensure that all marketed IVCTs have a reasonable assurance of both analytical and clinical validity.
14
In Vitro Diagnostics
The information that must be submitted to the FDA in order to obtain clearance or approval to market a new IVD varies depending on how the device is classified by the FDA. Medical devices are classified into one of three classes on the basis of the controls deemed by the FDA to be necessary to reasonably ensure their safety and effectiveness. Class I devices are subject to general controls, including labeling, and adherence to the FDA’s quality system regulations, which are device-specific good manufacturing practices. Class II devices are subject to premarket notification, general controls and sometimes special controls, including performance standards and post-market surveillance. Class III devices are subject to most of the previously identified requirements as well as to premarket approval. All Class I devices are exempt from premarket review; most Class II devices require 510(k) clearance, and all Class III devices must receive premarket approval before they can be sold in the United States. The payment of a fee, that is typically adjusted annually, to the FDA is usually required when a 510(k) notice or premarket approval application is submitted.
510(k) Premarket Notification
A 510(k) notification requires the sponsor to demonstrate that an IVD is substantially equivalent to another marketed device, termed a “predicate device”, that is legally marketed in the United States and for which a premarket approval application (“PMA”) was not required. A device is substantially equivalent to a predicate device if it has the same intended use and technological characteristics as the predicate; or has the same intended use but different technological characteristics, where the information submitted to the FDA does not raise new questions of safety and effectiveness and demonstrates that the device is at least as safe and effective as the legally marketed device.
Most 510(k)s do not require clinical data for clearance, but a minority will. Requests for additional data, including clinical data, will increase the time necessary to review the notice. If the FDA believes that the IVD is not substantially equivalent to a predicate device, it will issue a “Not Substantially Equivalent” letter and designate the device as a Class III device, which will require the submission and approval of a PMA before the new device may be marketed. Under certain circumstances, the sponsor may request the FDA to make a risk-based determination of the new device and reclassify the new device as a Class I or Class II device. The FDA continues to reevaluate the 510(k) pathway and process and the de novo process, and has taken what it describes as a risk-based approach to develop innovative regulatory policy to propose a more “contemporary” approach. We cannot predict what if any changes will occur or how they will affect our current or future products.
Premarket Approval
The PMA process is more complex, costly and time consuming than the 510(k) process. A PMA must be supported by more detailed and comprehensive scientific evidence, including clinical data, to demonstrate the safety and efficacy of the IVD for its intended purpose. If the device is determined to present a “significant risk,” the sponsor may not begin a clinical trial until it submits an investigational device exemption (“IDE”) to the FDA and obtains approval to begin the trial.
After the PMA is submitted, the FDA has 45 days to make a threshold determination that the PMA is sufficiently complete to permit a substantive review. If the PMA is complete, the FDA will file the PMA. The FDA is subject to a performance goal review time for a PMA that is 180 days from the date of filing, although in practice this review time is longer. Questions from the FDA, requests for additional data including additional clinical data and referrals to advisory committees may delay the process considerably. The total process may take several years and there is no guarantee that the PMA will ever be approved. Even if approved, the FDA may limit the indications for which the device may be marketed. Any changes to an approved medical device may require a supplemental PMA to be submitted and approved before the changed medical device may be marketed.
Any products sold by us pursuant to FDA clearances or approvals will be subject to pervasive and continuing regulation by the FDA, including record keeping requirements, reporting of adverse experiences with the use of the device and restrictions on the advertising and promotion of our products. Device manufacturers are required to register their establishments and list their devices with the FDA and are subject to periodic inspections by the FDA and certain state agencies. Noncompliance with applicable FDA requirements can result in, among other things, warning letters, fines, injunctions, civil penalties, recalls or seizures of products, total or partial suspension of production, refusal of the FDA to grant 510(k) clearance or PMA approval for new devices, withdrawal of 510(k) clearances and/or PMA approvals, civil penalties and criminal prosecution.
15
Regulation of Companion Diagnostic Devices
If a sponsor or the FDA believes that a diagnostic test is essential for the safe and effective use of a corresponding therapeutic product, the sponsor of the therapeutic product will typically work with a collaborator to develop an IVD companion diagnostic device. IVDs are regulated by the FDA as medical devices, and FDA issued a final guidance document in 2014, entitled “In Vitro Companion Diagnostic Devices” that is intended to assist companies developing in vitro companion diagnostic devices and companies developing therapeutic products that depend on the use of a specific in vitro companion diagnostic for the safe and effective use of the product. In the guidance, the FDA defined an IVD companion diagnostic device as a device that provides information that is essential for the safe and effective use of a corresponding therapeutic product. The FDA also noted that in some cases, if evidence is sufficient to conclude that the IVD companion diagnostic device is appropriate for use with a class of therapeutic products, the intended use/indications for use should name the therapeutic class, rather than each specific product within the class. In April 2020, FDA published a final guidance entitled, “Developing and Labeling In Vitro Companion Diagnostic Devices for a Specific Group or Class of Oncology Therapeutic Products” that expands on the idea of a class of therapeutic products introduced in the 2014 guidance. The new guidance describes considerations for the development and labeling of in vitro companion diagnostic devices to support the indicated uses of multiple drug or biological oncology products, when appropriate. The FDA expects that the therapeutic sponsor will address the need for an approved or cleared IVD companion diagnostic device in its therapeutic product development plan and that, in most cases, the therapeutic product and its corresponding IVD companion diagnostic will be developed contemporaneously. To that end, the FDA issued draft guidance on July 15, 2016 entitled “Principles for Codevelopment of an In Vitro Companion Diagnostic Device with a Therapeutic Product” to serve as a practical guide to assist therapeutic product sponsors and IVD sponsors in developing a therapeutic product and an accompanying IVD companion diagnostic.
The FDA indicated that it will apply a risk-based approach to determine the regulatory pathway for IVD companion diagnostic devices, as it does with all medical devices. This means that the regulatory pathway will depend on the level of risk to patients, based on the intended use of the IVD companion diagnostic device and the controls necessary to provide a reasonable assurance of safety and effectiveness.
If the companion diagnostic test will be used to make critical treatment decisions such as patient selection, treatment assignment, or treatment arm, it will likely be considered a significant risk device for which a clinical trial will be required. The sponsor of the IVD companion diagnostic device will be required to comply with the FDA’s IDE requirements that apply to clinical trials of significant risk devices. If the diagnostic test and the therapeutic drug are studied together to support their respective approvals, the clinical trial must meet both the IDE and IND requirements. We expect that any IVD companion diagnostic device developed for use with drug products will utilize the PMA pathway and that a clinical trial performed under an IDE will have to be completed before the PMA may be submitted.
We are developing companion diagnostic tests for use with drug products in development by pharmaceutical companies, such as our collaborations with pharmaceutical companies on PARP inhibitors for the treatment of ovarian, breast and other cancers. The FDA has also introduced the concept of a complementary diagnostic that it defines as a test that is not required but which provides significant information about the use of a drug. A complementary test can help guide treatment strategy and identify which patients are likely to derive the greatest benefit from therapy, and if approved by the FDA information regarding the IVD will be included in the therapeutic product labeling. Although the FDA has not yet issued any written guidance regarding complementary diagnostics, it has already approved some complementary diagnostics, including a supplementary premarket approval for BRACAnalysis CDx and myChoice CDx as complementary diagnostic tests in ovarian cancer patients associated with enhanced progression-free survival (PFS) when used with the PARP inhibitor Zejula™ (niraparib).
16
In December 2014, we first obtained premarket approval for BRACAnalysis CDx, which is used as a companion diagnostic test to identify ovarian cancer patients who may benefit from AstraZeneca’s PARP inhibitor Lynparza™ (olaparib). Since then, other indications for BRACAnalysis CDx in ovarian, breast, prostate and pancreatic cancer have received supplemental PMA approval as a companion diagnostic for Lynparza. The myChoice CDx test has also received approvals as a companion diagnostic test. The premarket approval process is a complex, costly and time consuming procedure. Approvals must be supported by valid scientific evidence, submitted as part of a PMA, which typically requires extensive data, including quality technical, preclinical, clinical and manufacturing data to demonstrate to the FDA’s satisfaction the safety and effectiveness of the companion diagnostic. We are currently collaborating with several pharmaceutical companies, including AstraZeneca, Merck, Pfizer, GSK, AbbVie, and others for additional indications and geographical commercialization opportunities for BRACAnalysis CDx and myChoice CDx, to evaluate the use of several of our tests as companion diagnostics with other drugs.
After a medical device is placed on the market, numerous regulatory requirements apply. These include:
•compliance with the FDA’s Quality System Regulation (“QSR”), which requires manufacturers to follow stringent design, testing, control, documentation, record maintenance, including maintenance of complaint and related investigation files, and other quality assurance controls during the manufacturing process;
•labeling regulations, which prohibit the promotion of products for uncleared, or unapproved uses, or “off-label” uses, and impose other restrictions on labeling; and
•medical device reporting obligations, which require that manufacturers investigate and report to the FDA adverse events, including deaths, or serious injuries that may have been or were caused by a medical device and malfunctions in the device that would likely cause or contribute to a death or serious injury if it were to recur.
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include sanctions, including but not limited to, warning letters; fines, injunctions, and civil penalties; recall or seizure of the device; operating restrictions, partial suspension or total shutdown of production; refusal to grant 510(k) clearance or approval of PMAs of new devices; withdrawal of clearance or approval; and civil or criminal prosecution.
Products intended for use in in vitro diagnostic applications require regulatory approvals in many other countries and geographic areas, some of which also provide for approval of companion diagnostics. For example, in the European Union, in vitro diagnostic medical devices have been regulated under the European Union Directive 98/79/EC (the “Directive”), since 2003, and all products and kits used for in vitro diagnostic applications must be compliant with the Directive. IVDs have not been subject to pre-market authorization under the Directive, but instead they had to comply with essential requirements based on conformity with harmonized standards. The majority of IVDs have been self-certified by manufacturers which can place a CE mark on most products to show that they meet the conformity assessment. EU Member States must ensure that IVDs are only placed on the market if they conform to the requirements, and must ensure the free movement of such devices in the internal market. Member States will designate independent organizations, or Notified Bodies, that ensure that a conformity assessment is carried out for devices. These Notified Bodies may carry out inspections of certain manufacturers, and manufacturers must report any incident causing death to, or damaging the health of, a patient to the competent authorities.
In May 2022, the Directive will be replaced by the In Vitro Diagnostic Device Regulation (IVDR) European Union (EU) 2017/746 that was published in May 2017, and given a 5-year transition period until its implementation on May 27, 2022. Unlike the Directive that specifies certain results that must be achieved by each Member State and permits each Member State to decide how to transpose the Directive into national law, the IVDR has binding legal force throughout every Member State and it will become effective on a set date in all the Member States. The major goals of the IVDR are to standardize diagnostic procedures within the EU, increase reliability of diagnostic analysis and enhance patient safety. Under the IVDR, as enacted by the European Commission (EC), in vitro diagnostics will be subject to additional legal regulatory requirements after it comes into full effect. Among other things, the IVDR introduces a new risk classification system and requirements for conformity assessments. Products already certified by a Notified Body may remain on the market until May 25, 2024 under some conditions including fulfillment of specific requirements in the IVDR, but ultimately most products will have to be approved. Compliance with the IVDR may be expensive and time-consuming. Manufacturers will need to provide more data to demonstrate that a device performs safely and effectively. As noted above, the vast majority of IVDs under the Directive are self-certified, so many device manufacturers have not previously been subject to the Notified Body audits that will occur under the IVDR and will have to revise their Quality Management System (QMS) and Technical Documentation which will be reviewed. There will also be a greater emphasis on post-market surveillance and submission of post-market performance follow-up reports.
17
UK
The UK's withdrawal from the EU will have major ramifications for IVD manufacturers, that will, among other things, have to follow new procedures that will apply in the UK including appointment of UK Responsible Persons rather than relying on European Authorized Representatives to manage their compliance efforts in the UK.
The UK Medicine and Healthcare Products Regulatory Agency (MHRA) issued new guidance on how the country will regulate IVDs after January 1, 2021, According to MHRA, IVDs in the future will require certification in the UK, which is defined as England, Scotland, and Wales, while companies will still be able to sell tests in Northern Ireland under existing EU IVD regulations.
As described in the guidance, MHRA will continue to recognize CE marks until June 30, 2023. Companies wishing to place IVDs on the UK market will require registration with MHRA after January 1, 2021, but will still be able to sell CE-IVD marked products for the next two-and-a-half years. After July 1, 2023, companies selling in the UK will have to obtain a new marking called a UK Conformity Assessed mark, or UKCA. This mark will not be automatically recognized in EU countries, meaning that companies that wish to sell in the UK and the EU will have to seek both a UKCA and CE-IVD mark in the future. We anticipate that more information about the new UK requirements will become available in the near future.
Japan
IVDs are regulated in Japan by the Pharmaceutical and Medical Devices Agency, or PMDA, and are assigned to one of three classes depending on the perceived level of risk. Those in the least risky class may be registered and marketed after filing a pre-market submission, while those in the middle class are subject to pre-market certification by a registered certification body. The riskiest IVDs must be approved. Submissions may be made only by marketing authorization holders, which must satisfy specific requirements.
Significant revisions to Japanese regulations of medical devices, IVDs and other healthcare products are on-going, with phased implementations of new and updated requirements planned through 2022. The first round of changes to Japan’s Pharmaceuticals and Medical Devices Act took effect September 1, 2020, and additional revisions will come into force in August 2021 and December 2022. Some of those changes will affect IVDs, including the ability to qualify for fast track designation.
Other Regulatory Requirements
Our laboratories are subject to federal, state and local regulations relating to the handling and disposal of regulated medical waste, hazardous waste and biohazardous waste, including chemical, biological agents and compounds, blood and bone marrow samples and other human tissue. Typically, we use outside vendors who are contractually obligated to comply with applicable laws and regulations to dispose of such waste. These vendors are licensed or otherwise qualified to handle and dispose of such waste.
HIPAA and other privacy laws
The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which applies to health plans, healthcare clearing houses, and healthcare providers that conduct certain healthcare transactions electronically (“Covered Entities”) contains provisions that address the privacy of health data, the security of health data, the standardization of identifying numbers used in the healthcare system and the standardization of certain healthcare transactions. The privacy regulations protect health information by limiting its use and release, giving patients the right to access their medical records and limiting most disclosures of health information to the minimum amount necessary to accomplish an intended purpose. The HIPAA security standards require the adoption of administrative, physical, and technical safeguards and the adoption of written security policies and procedures.
18
On February 17, 2009, Congress enacted the Health Information Technology for Economic and Clinical Health Act (“HITECH”), which expanded and strengthened HIPAA, created new targets for enforcement, imposed new penalties for noncompliance and established new breach notification requirements for Covered Entities. Under HITECH’s breach notification requirements, Covered Entities must report breaches of protected health information that has not been encrypted or otherwise secured in accordance with guidance from the Secretary of the U.S. Department of Health and Human Services (the “Secretary”). Required breach notices must be made as soon as is reasonably practicable, but no later than 60 days following discovery of the breach. Reports must be made to affected individuals and to the Secretary and, in some cases depending on the size of the breach, they must be reported through local and national media. Breach reports can lead to investigation, enforcement, civil monetary penalties and civil litigation, including class action lawsuits and enforcement by state.
We are currently subject to the HIPAA regulations and maintain an active compliance program that is designed to identify security incidents and other issues in a timely fashion and enable us to remediate, mitigate harm or report if required by law. However, even if we make required reports and remediate on a timely basis, we may still be subject to penalties for the underlying breach.
In addition to the federal privacy and security regulations, there are a number of state laws regarding the privacy and security of health information and personal data that are applicable to our clinical laboratories. Many states have also implemented genetic testing and privacy laws imposing specific patient consent requirements and protecting test results by strictly limiting the disclosure of those results. State requirements are particularly stringent regarding predictive genetic tests, due to the risk of genetic discrimination against healthy patients identified through testing as being at a high risk for disease. We believe that we have taken the steps required of us to comply with health information privacy and security statutes and regulations, including genetic testing and genetic information privacy laws in all jurisdictions, both state and federal. However, these laws constantly change, and we may not be able to maintain compliance in all jurisdictions where we do business. Failure to maintain compliance, or changes in state or federal laws regarding privacy or security could result in civil and/or criminal penalties, significant reputational damage and could have a material adverse effect on our business.
The General Data Protection Regulation (“GDPR”), which applies to all EU member states from May 25, 2018, also applies to some of our operations. The GDPR is discussed in more detail elsewhere in this report.
We are subject to laws and regulations related to the protection of the environment, the health and safety of employees and the handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials. For example, the U.S. Occupational Safety and Health Administration (“OSHA”) has established extensive requirements relating specifically to workplace safety for healthcare employers in the U.S. This includes requirements to develop and implement multi-faceted programs to protect workers from exposure to blood-borne pathogens, including preventing or minimizing any exposure through needle stick injuries. For purposes of transportation, some biological materials and laboratory supplies are classified as hazardous materials and are subject to regulation by one or more of the following agencies: the U.S. Department of Transportation, the U.S. Public Health Service, the United States Postal Service, the Office of Foreign Assets Control, and the International Air Transport Association. We generally use third-party vendors to dispose of regulated medical waste, hazardous waste and radioactive materials and contractually require them to comply with applicable laws and regulations.
Transparency Laws and Regulations
A federal law known as the Physician Payments Sunshine Act (the “Sunshine Act”) requires medical device manufacturers to track and report to the federal government certain payments and other transfers of value made to covered recipients, which is defined as physicians, physician assistants, nurse practitioners, clinical nurse specialists, certified registered nurse anesthetists, and certified nurse-midwives who are not bona fide employees of the manufacturer, as well as teaching hospitals, and ownership or investment interests held by physicians and their immediate family members. Manufacturers must report data for the previous calendar year by the 90th day of the then-current calendar year. CMS then publishes the data on a publicly available website no later than June 30th. There are also state “sunshine” laws that require manufacturers to provide reports to state governments on pricing and marketing information. Several states have enacted legislation requiring medical device manufacturers to, among other things, establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales and marketing activities, and such laws may also prohibit or limit certain other sales and marketing practices. These laws may adversely affect our sales, marketing, and other activities by imposing administrative and compliance burdens on us. If we fail to track and report if and the extent required by these laws or to otherwise comply with these laws, we could be subject to the penalty provisions of the pertinent state and federal authorities.
19
Reimbursement and Billing
Reimbursement and billing for diagnostic services is highly complex. Laboratories must bill various payors, such as private third-party payors, including managed care organizations (“MCO”), and state and federal health care programs, such as Medicare and Medicaid, and each may have different billing requirements. Additionally, the audit requirements we must meet to ensure compliance with applicable laws and regulations, as well as our internal compliance policies and procedures, add further complexity to the billing process. Other factors that complicate billing include:
•variability in coverage and information requirements among various payors;
•patient financial assistance programs;
•missing, incomplete or inaccurate billing information provided by ordering physicians;
•billings to payors with whom we do not have contracts;
•disputes with payors as to which party is responsible for payment; and
•disputes with payors as to the appropriate level of reimbursement.
Depending on the reimbursement arrangement and applicable law, the party that reimburses us for our services may be:
•a third party who provides coverage to the patient, such as an insurance company or MCO;
•a state or federal healthcare program; or
•the patient.
Presently, approximately 65% of our revenue comes from private third-party payors.
Federal and State Fraud and Abuse Laws
A variety of state and federal laws prohibit fraud and abuse involving state and federal healthcare programs, such as Medicare and Medicaid. These laws are interpreted broadly and enforced aggressively by various state and federal agencies, including CMS, the Department of Justice, the Office of Inspector General for the Department of Health and Human Services (“OIG”), and various state agencies. In addition, the Medicare and Medicaid programs increasingly use a variety of contractors to review claims data and to identify improper payments as well as fraud and abuse. Any overpayments must be repaid within 60 days of identification unless a favorable decision is obtained on appeal. In some cases, these overpayments can be used as the basis for an extrapolation, by which the error rate is applied to a larger set of claims, and which can result in even higher repayments.
Anti-Kickback Laws
The Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for or recommending of an item or service that is reimbursable, in whole or in part, by a federal health care program. “Remuneration” is broadly interpreted by some agencies to include anything of monetary value, such as, for example, cash payments, gifts or gift certificates, discounts, or the furnishing of services, supplies or equipment. The Anti-Kickback Statute can be interpreted broadly to prohibit many arrangements and practices that are lawful in businesses outside of the health care industry.
Recognizing the potential breadth of interpretation of the Anti-Kickback Statute and the fact that it may technically prohibit many innocuous or beneficial arrangements within the health care industry, the OIG has issued a series of regulations, or safe harbors intended to protect such arrangements. Compliance with all requirements of a safe harbor immunizes the parties to the business arrangement from prosecution under the Anti-Kickback Statute. The failure of a business arrangement to fit within a safe harbor does not necessarily mean that the arrangement is illegal or that the OIG will pursue prosecution. Still, in the absence of an applicable safe harbor, a violation of the Anti-Kickback Statute may occur even if only one purpose of an arrangement is to induce referrals. The penalties for violating the Anti-Kickback Statute can be severe. These sanctions include criminal and civil penalties, imprisonment and possible exclusion from federal health care programs. Many states have adopted laws similar to the Anti-Kickback Statute, and some apply to items and services reimbursable by any payor, including private third-party payors.
20
In addition, in October 2018, the Eliminating Kickbacks in Recovery Act of 2018 (“EKRA”), was enacted as part of the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act (the “SUPPORT Act”). EKRA is an all-payor anti-kickback law that makes it a criminal offense to pay any remuneration to induce referrals to, or in exchange for, patients using the services of a recovery home, a substance use clinical treatment facility, or laboratory. Although it appears that EKRA was intended to reach patient brokering and similar arrangements to induce patronage of substance use recovery and treatment, the language in EKRA is broadly written. Further, certain of EKRA’s exceptions, such as the exception applicable to relationships with employees that effectively prohibits volume-based incentive compensation, are inconsistent with the Anti-Kickback Statute regulations, which permit payment of employee incentive compensation, a practice that is common in the industry. Significantly, EKRA permits the U.S. Department of Justice to issue regulations clarifying EKRA’s exceptions or adding additional exceptions, but such regulations have not yet been issued. Laboratory industry stakeholders are reportedly seeking clarification regarding EKRA’s scope and/or amendments to its language. Because EKRA is a new law, there is no agency guidance or court precedent to indicate how and to what extent it will be applied and enforced.
Physician Self-Referral Bans
The federal ban on physician self-referrals, commonly known as the Stark Law, prohibits, subject to certain exceptions, physician referrals of Medicare patients to an entity providing certain designated health services, which include laboratory services, if the physician or an immediate family member of the physician has any financial relationship with the entity. Several Stark Law exceptions are relevant to arrangements involving clinical laboratories, including but not limited to: (1) fair market value compensation for the provision of items or services; (2) payments by physicians to a laboratory for clinical laboratory services; (3) certain space and equipment rental arrangements that satisfy certain requirements; and (4) personal services arrangements. Penalties for violating the Stark Law include the return of funds received for all prohibited referrals, fines, civil monetary penalties and possible exclusion from federal health care programs. In addition to the Stark Law, many states have their own self-referral bans, which may extend to all self-referrals, regardless of the payor.
State and Federal Prohibitions on False Claims
The federal False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to the federal government. Under the False Claims Act, a person acts knowingly if he or she has actual knowledge of the information or acts in deliberate ignorance or in reckless disregard of the truth or falsity of the information. Specific intent to defraud is not required. The qui tam provisions of the False Claims Act allow a private individual to bring an action on behalf of the federal government and to share in any amounts paid by the defendant to the government in connection with the action. Penalties include payment of up to three times the actual damages sustained by the government, plus civil penalties of between $5,500 and $11,000 for each false claim, as well as possible exclusion from federal health care programs. However, the civil penalty amounts are adjusted annually for inflation. For civil penalties assessed after June 19, 2020, whose associated violations occurred after November 2, 2015, the civil penalty amount ranges between $11,665 and $23,331 per claim. In addition, various states have enacted similar laws modeled after the False Claims Act that apply to items and services reimbursed under Medicaid and other state health care programs, and, in several states, such laws apply to claims submitted to any payor.
Civil Monetary Penalties Law
The federal Civil Monetary Penalties Law (the “CMP Law”), prohibits, among other things, (1) the offering or transfer of remuneration to a Medicare or state health care program beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or a state health care program, unless an exception applies; (2) employing or contracting with an individual or entity that the provider knows or should know is excluded from participation in a federal health care program; (3) billing for services requested by an unlicensed physician or an excluded provider; and (4) billing for medically unnecessary services. The penalties for violating the CMP Law include exclusion, substantial fines, and payment of up to three times the amount billed, depending on the nature of the offense.
21
International regulations
We market, directly or through distributors, some of our tests outside of the United States and are subject to foreign regulatory requirements governing laboratory licensure, human clinical testing, use of tissue, privacy and data security, and marketing approval for our tests. These requirements vary by jurisdiction, differ from those in the United States and may require us to implement additional compliance measures or perform additional pre-clinical or clinical testing. For example, the In Vitro Diagnostic Medical Devices (2017/746/EU) (“IVDR”) will replace the existing In Vitro Diagnostic Medical Devices Directive (98/79/EC) (“IVDD”) in the European Union (“EU”). The IVDR was published in May 2017, marking the start of a five-year period of transition from the IVDD. During the transitional period the IVDR will come into force gradually, starting with the provisions related to the designation of Notified Bodies and the ability of manufacturers to apply for new certificates under the IVDR. The transitional period will end on May 26, 2022, the “Date of Application” of the Regulation. From that point the IVDR will apply fully. The EU has also implemented the GDPR, which requires us to meet new and more stringent requirements regarding the handling of personal data about European Union residents. In many countries outside of the United States, coverage, pricing and reimbursement approvals are also required. We are also required to maintain accurate information on and control over sales and distributors’ activities that may fall within the purview of the Foreign Corrupt Practices Act, its books and records provisions and its anti-bribery provisions.
Available Information
We are a Delaware corporation with our principal executive offices located at 320 Wakara Way, Salt Lake City, Utah 84108. Our telephone number is (801) 584-3600 and our website address is www.myriad.com. We make available free of charge through the Investor Relations section of our website our Corporate Code of Conduct and Ethics, our Audit Committee and other committee charters and our other corporate governance policies, as well as our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, current reports on Form 8-K and all amendments to those reports as soon as reasonably practicable after such material is electronically filed with or furnished to the Securities and Exchange Commission. The Securities and Exchange Commission maintains an internet site (http://www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the Securities and Exchange Commission. We include our website address in this Transition Report on Form 10-K only as an inactive textual reference and do not intend it to be an active link to our website.
22
Item 1A. RISK FACTORS
Risk Factors Summary
The following is a summary of the principal risks that could adversely affect our business, operations, and financial results:
Risks Related to Our Business and Our Strategy
•Our financial condition and results of operations could be further adversely affected by the ongoing coronavirus outbreak.
•We may not be able to generate sufficient revenue from our existing tests or develop new tests to be profitable.
•We may not be able to maintain revenue growth or operate our business on a profitable basis.
•If we do not continue to generate sufficient revenue from sales of our molecular diagnostic tests and are unable to secure additional funding, we may have to reduce our operations.
•We are subject to debt covenants that impose operating and financial restrictions on us and if we are not able to comply with them, it could have a material adverse impact on our operations and liquidity.
•If our current operating plan changes and we find that our existing capital resources will not meet our needs, we may find it necessary to raise additional funding, which may not be available.
•We may acquire technologies, assets or other businesses that could cause us to incur significant expense and expose us to a number of unanticipated operational and financial risks, which could adversely affect our financial condition, results of operations and business prospects.
•If we were successfully sued for product liability, we could face substantial liabilities that exceed our resources.
•We are dependent on our information technology and telecommunications systems, and any failure of these systems could harm our business.
•Security breaches, loss of data and other disruptions could compromise sensitive information related to our business, prevent us from accessing critical information or expose us to liability, which could adversely affect our business and our reputation.
•We may be adversely impacted if we are unable to successfully implement new systems or unable to adapt systems to our change in fiscal year-end.
•We have identified a material weakness in our internal control over accounting for intercompany transactions, foreign currency exchanges and foreign currency translation related to our international subsidiaries and such weakness led to a conclusion that our internal control over financial reporting and disclosure controls and procedures were not effective as of December 31, 2020. Our ability to remediate the material weakness, our discovery of additional weaknesses, and our inability to achieve and maintain effective disclosure controls and procedures and internal control over financial reporting, could adversely affect our results of operations, our stock price and investor confidence in our company.
•Our business involves environmental risks that may result in liability for us.
•Changes in health care policy could increase our costs, decrease our revenues and impact sales of and reimbursement for our tests.
•We face risks associated with currency exchange rate fluctuations, which could adversely affect our operating results.
Risks Related to Commercialization of Our Tests, Our Services and Test Candidates
•Our pharmaceutical testing services customers may reduce the amount of testing they conduct through us.
•Our molecular diagnostic and companion diagnostic tests in development may never achieve significant commercial market acceptance.
•If we do not compete effectively with scientific and commercial competitors, we may not be able to successfully commercialize our tests.
•Our international business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States.
•Foreign governments may impose reimbursement standards, which may adversely affect our future profitability.
•International data protection laws and regulations may restrict our activities and increase our costs.
•We rely on a single laboratory facility to process each of our molecular diagnostic tests in the United States and Europe a single laboratory facility to perform our pharmaceutical and clinical services. Failure to maintain the operations of these laboratories in compliance with applicable regulations would seriously harm our business.
•We depend on a limited number of third parties for some of our supplies of equipment and reagents. If these supplies become unavailable, then we may not be able to successfully perform our research or operate our business on a timely basis or at all.
23
•If our current research collaborators or scientific advisors terminate their relationships with us or develop relationships with a competitor, our ability to discover genes, proteins, and biomarkers, and to validate and commercialize molecular diagnostic and companion diagnostic tests could be adversely affected.
•If we fail to retain our key personnel and hire, train and retain qualified employees and consultants, we may not be able to successfully continue our business.
Risks Related to Our Intellectual Property
•If we are not able to protect our proprietary technology, others could compete against us more directly, which would harm our business.
•If we were sued for patent infringement by third parties, we might incur significant costs and delays in test introduction.
•We may be unable to adequately prevent disclosure of trade secrets, proprietary databases, and other proprietary information.
•If we fail to comply with our obligations under license or technology agreements with third parties, we could lose license rights that are critical to our business.
•We may be subject to claims that we or our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Risks Related to Government Regulation
•If we fail to comply with the complex federal, state, local and foreign laws and regulations that apply to our business, we could suffer severe consequences that could materially and adversely affect our operating results and financial condition.
•FDA regulation of our industry generally or our tests specifically could be disruptive to our business.
•Failure to comply with laws and regulations related to submission of claims for our services could result in significant monetary damages and penalties and exclusion from the Medicare and Medicaid programs and corresponding foreign reimbursement programs.
•We are currently subject to government investigation(s), the unfavorable outcome of which may have a material adverse effect on our financial condition, results of operations and cash flows.
•Our business could be harmed by the loss, suspension, or other restriction on a license, certification, or accreditation, or by the imposition of a fine or penalties, under CLIA, its implementing regulations, or other state, federal and foreign laws and regulations affecting licensure or certification, or by future changes in these laws or regulations.
•Changes in the way that the FDA regulates tests performed by laboratories like ours could result in delay or additional expense in offering our tests and tests that we may develop in the future.
•Companion and complementary diagnostic tests require FDA approval and we may not be able to secure such approval in a timely manner or at all.
•If the government and third-party payors fail to provide coverage and adequate payment for our tests and future tests, if any, our revenue and prospects for profitability will be harmed.
•Our business could be adversely impacted by our failure or the failure of physicians to comply with any new ICD Code Set.
Risks Related to Our Common Stock
•Our stock price is highly volatile, and our stock may lose all or a significant part of its value.
•Anti-takeover provisions of Delaware law, provisions in our charter and bylaws and re-adoption of our stockholders’ rights plan, or poison pill, could make a third-party acquisition of us difficult.
Risks Related to Our Business and Our Strategy
Our financial condition and results of operations could be further adversely affected by the ongoing coronavirus outbreak.
Any outbreak of contagious diseases, such as COVID-19, or other adverse public health developments, could have a material and adverse effect on our business operations. For example, government public health officials may place additional restrictions to curb the spread of COVID-19, further limiting patients' access to our services, which may impede our progress in returning to profitability. Such adverse effects could include diversion or prioritization of healthcare resources away from the conduct of genetic testing, disruptions or restrictions on the ability of laboratories to process our tests, and delays or difficulties in patients accessing our tests, including those resulting from an inability to travel as a result of quarantines or other restrictions resulting from COVID-19.
24
As COVID-19 continues to affect individuals and businesses around the globe, we will likely experience disruptions that could severely impact our business, including:
•decreased volume of testing as a result of disruptions to healthcare providers and limitations on the ability of providers to administer tests;
•disruptions or restrictions on the ability of our, our collaborators’, or our suppliers’ personnel to travel, and could result in temporary closures of our facilities or the facilities of our collaborators or suppliers;
•limitations on employee resources that would otherwise be focused on the development of our products, processing our diagnostic tests, and the conduct of our clinical trials, including because of sickness of employees or their families or requirements imposed on employees to avoid contact with large groups of people; and
•delays in necessary interactions with local regulators, ethics committees and other important agencies and contractors due to limitations in employee resources or forced furlough of government employees.
In addition, the continued spread of COVID-19 globally could adversely affect our manufacturing and supply chain. Parts of our direct and indirect supply chain are located overseas and both international and domestic components may be subject to disruption as a result of COVID-19 and ongoing responses to it. Additionally, our results of operations could be adversely affected to the extent that COVID-19 or any other public health emergency harms our business or the economy in general either domestically or in any other region in which we do business. The extent to which COVID-19 affects our operations will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the outbreak, new information that may emerge concerning the severity of COVID-19 and the actions to contain COVID-19 or treat its impact, among others, which could have an adverse effect on our business and financial condition.
We may not be able to generate sufficient revenue from our existing tests or develop new tests to be profitable.
We believe our future success is dependent upon our ability to successfully market our existing molecular diagnostic tests to additional patients within the United States, to expand into new markets within and outside the United States, and to develop and commercialize new molecular diagnostic and companion diagnostic tests. However, we may not be able to generate sufficient revenue from our existing tests and in launching and commercializing our new tests. The demand for our existing molecular diagnostic tests may decrease or may not continue to increase at historical rates due to sales of new tests that may replace our existing product portfolio, or for other reasons. For example, because most of our molecular diagnostic tests are only utilized once per patient, we will need to sell our services through physicians to new patients or develop new molecular diagnostic tests in order to continue to generate revenue. Our pipeline of new molecular diagnostic and companion diagnostic test candidates is in various stages of development and may take several more years to develop and must undergo extensive clinical validation. We may be unable to discover or develop any additional molecular diagnostic or companion diagnostic tests through the utilization of our technologies or technologies we license or acquire from others. Even if we develop tests or services for commercial use, we may not be able to develop tests or services that:
•meet applicable regulatory standards, in a timely manner or at all;
•successfully compete with other technologies and tests;
•avoid infringing the proprietary rights of others;
•are adequately reimbursed by third-party payors;
•can be performed at commercial levels or at reasonable cost; or
•can be successfully marketed.
We must generate significant revenue to maintain profitability. Even if we succeed in marketing our existing molecular diagnostic tests to physicians for use in new patients and in developing and commercializing any additional molecular diagnostic tests and companion diagnostic tests, we may not be able to generate sufficient revenue and we may not be profitable.
We may not be able to maintain revenue growth or operate our business on a profitable basis.
We may not be able to generate revenue growth or maintain existing revenue levels. Historically, our molecular diagnostic business has operated profitably providing a cash contribution to our funding and operational needs. We may not, however, be able to operate our molecular diagnostic business on a profitable basis in the future. Potential events or factors that may have a significant impact on our ability to sustain revenue growth and obtain profitability for our molecular diagnostic business include the following:
25
•increased costs of reagents and other consumables required for molecular diagnostic testing;
•increased personnel and facility costs;
•our inability to hire competent, trained staff, including laboratory directors required to review and approve all reports we issue in our molecular diagnostic business, and sales personnel;
•our inability to obtain necessary equipment or reagents to perform molecular diagnostic testing;
•our inability to increase production capacity as demand increases;
•our inability to expand into new markets within or outside the United States;
•the efforts of third-party payors to limit or decrease the amounts that they are willing to pay for our tests, recoup amounts already paid, or institute burdensome administrative requirements for reimbursement, such as prior authorization requirements;
•increased licensing or royalty costs, and our ability to maintain and enforce the intellectual property rights underlying our tests and services;
•changes in intellectual propriety law applicable to our patents or enforcement in the United States and foreign countries;
•potential obsolescence of our tests;
•our inability to increase commercial acceptance of our molecular diagnostic tests;
•increased competition and loss of market share;
•increased regulatory requirements; and
•material litigation costs and judgments.
If we do not continue to generate sufficient revenue from sales of our molecular diagnostic tests and are unable to secure additional funding, we may have to reduce our operations.
While we anticipate that our existing cash, cash equivalents and marketable securities and expected net cash to be generated from sales of our molecular diagnostic tests and pharmaceutical and clinical services will be sufficient to fund our current operations for the foreseeable future, changes could occur that would consume available capital resources more quickly than we currently expect and we may need or want to raise additional financing. On December 23, 2016, we entered into a senior secured revolving credit facility as borrower, with the lenders from time to time party thereto, which was amended on July 31, 2018, May 1, 2020 and February 22, 2021 (the "Amended Facility"). If we are unable to secure additional funding, we may be unable to repay our Amended Facility when it becomes due, or in the event of a debt covenant default, and be required to reduce research and development projects, limit sales and marketing activities, scale back our expansion efforts within or outside the United States, reduce headcount or potentially even discontinue operations. Our future capital requirements will depend on many factors that are currently unknown to us, including:
•the scope, progress, results and cost of development, clinical testing and pre-market studies of any new molecular diagnostic tests that we may discover or acquire;
•the progress, results, and costs to develop additional molecular diagnostic tests;
•the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our current issued patents, and defending intellectual property-related claims;
•our ability to enter into collaborations, licensing or other arrangements favorable to us;
•the costs of acquiring technologies or businesses, and our ability to successfully integrate and achieve the expected benefits of our business development activities and acquisitions;
•the progress, cost and results of our international efforts;
•the costs of expanding our sales and marketing functions and commercial operation facilities in the United States and in new markets;
•the costs, timing and outcome of any litigation against us; and
•the costs to satisfy our current and future obligations.
We are subject to debt covenants that impose operating and financial restrictions on us and if we are not able to comply with them, it could have a material adverse impact on our operations and liquidity.
Covenants in the Amended Facility impose operating and financial restrictions on us. These restrictions may prohibit or place limitations on, among other things, our ability to incur additional indebtedness, create certain types of liens, and complete mergers, consolidations, or change in control transactions. Under the Amended Facility, a change in control of the Company, which means that a stockholder or a group of stockholders is or becomes the beneficial owner, directly or indirectly, of more than 35% of the total voting power of the voting stock of the Company, would require mandatory prepayment of the outstanding debt. The Amended Facility may also prohibit or place limitations on our ability to sell assets, pay dividends or provide other distributions to stockholders. These restrictions could also limit our ability to take advantage of business opportunities.
26
We must maintain specified leverage and interest ratios measured as of the end of each applicable quarter as financial covenants in the Amended Facility. The Amended Facility, through Amendment No. 2 entered into on May 1, 2020 and Amendment No. 3 entered into on February 22, 2021, modified compliance with the leverage covenant and the interest coverage ratio covenant, which were waived through March 31, 2022, and added a minimum liquidity covenant. If we are unable to improve our results of operations, it is possible that we could be in violation of certain financial covenants contained in the Amended Facility in the future. If we are unable to comply with the covenants and ratio in the Amended Facility, we may be in default under the agreement. A default would result in an increase in the rate of interest and limits on our ability to incur certain additional indebtedness and it could potentially cause the loan repayment to be accelerated, any of which could have a material adverse impact on our operations and liquidity.
If our current operating plan changes and we find that our existing capital resources will not meet our needs, we may find it necessary to raise additional funding, which may not be available.
We anticipate that our existing capital resources and expected net cash to be generated from sales of our molecular diagnostic tests will enable us to maintain our currently planned operations for the foreseeable future. However, we base this expectation on our current operating plan, which may change. We have incurred, and will continue to incur, significant costs in the discovery, development and marketing of current and prospective molecular diagnostic and companion diagnostic tests. Our ongoing efforts to develop tests and expand our business, which may be through internally developed products, in-licensing and mergers and acquisitions, will require substantial cash resources. If, due to changes in our current operating plan, adequate funds are not available, we may be required to raise additional funds. Sources of potential additional capital resources may include, but are not limited to, public or private equity financings, expanding or supplementing our Amended Facility, or selling convertible or non-convertible debt securities. This additional funding, if necessary, may not be available to us on reasonable terms, or at all. If we issue shares of stock or other securities to acquire new companies or technologies, the ownership interests of our existing stockholders may be significantly diluted.
Because of our potential long-term capital requirements, we may access the public or private equity or debt markets whenever conditions are favorable, even if we do not have an immediate need for additional capital at that time. Under Securities and Exchange Commission rules, we currently qualify as a well-known seasoned issuer, or WKSI, and can at any time file a registration statement registering securities to be sold to the public which would become effective upon filing. If additional funds are raised by issuing equity securities, existing stockholders may suffer significant dilution. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through collaborations, strategic alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or tests or grant licenses on terms that are not favorable to us.
We may acquire technologies, assets or other businesses that could cause us to incur significant expense and expose us to a number of unanticipated operational and financial risks, which could adversely affect our financial condition, results of operations and business prospects.
In addition to organic growth, we intend to continue to pursue growth through the acquisition of technology, assets or other businesses that may enable us to enhance our technologies and capabilities, expand our geographic market, add experienced management personnel and increase our test offerings. For example, in July 2018, we acquired Counsyl, Inc. and believe the acquisition allowed for greater entry into the high-growth reproductive testing market, with the ability to become a leader in women’s health genetic testing. However, these acquisitions may not achieve profitability or generate a positive return on our investment. Additionally, we may be unable to implement our growth strategy if we cannot identify suitable acquisition candidates, reach agreement on potential acquisitions on acceptable terms, successfully integrate personnel or assets that we acquire or for other reasons. Additionally, we may experience increased expenses, distraction of our management, personnel and customer uncertainty. Our acquisition efforts may involve certain risks, including:
•we may have difficulty integrating operations and systems;
•key personnel and customers of the acquired company may terminate their relationships with the acquired company as a result of the acquisition;
•we may not be successful in launching new molecular diagnostic tests or companion diagnostic tests, or if those tests are launched, they may not prove successful in the marketplace;
•we may experience additional financial and accounting challenges and complexities in areas such as tax planning and financial reporting;
27
•we may assume or be held liable for risks and liabilities, including for legal, compliance, recoupment, and environmental-related costs and liabilities, as a result of our acquisitions, some of which we may not discover during our due diligence;
•we may incur significant additional operating expenses;
•we may experience possible inconsistencies in the standards, controls, procedures, policies and compensation structures;
•we may encounter risks and limitations on our ability to consolidate corporate and administrative infrastructures of the two companies;
•our ongoing business may be disrupted or receive insufficient management attention; and
•we may not be able to realize synergies, the cost savings or other financial and operational benefits we anticipated, or such synergies, savings or benefits may take longer than we expected.
The process of negotiating acquisitions and integrating acquired tests, services, technologies, personnel or businesses might result in operating difficulties and expenditures and might require significant management attention that would otherwise be available for ongoing development of our business, whether or not any such transaction is ever consummated. Moreover, we might never realize the anticipated benefits of any acquisition such as increase in our scale, diversification, cash flows and operational efficiency and meaningful accretion to our diluted earnings per share. Future acquisitions could result in the use of our available cash and marketable securities, potentially dilutive issuances of equity securities, the need to incur additional debt, contingent liabilities, or impairment expenses related to goodwill, and impairment or amortization expenses related to other intangible assets, which could harm our financial condition. In addition, if we are unable to integrate any acquired businesses, tests or technologies effectively, our business, financial condition and results of operations may be materially adversely affected.
On the other hand, we may seek to divest assets, including but not limited to large capital equipment, diagnostic tests, intellectual property, business units, or corporate affiliates. For example, the Company announced in the last quarter, its intention to seek strategic alternatives for its Myriad RBM, myPath Melanoma, and Vectra businesses. The price we are able to command for such assets may not be high and, in some cases, may be lower than the amount we invested in or paid for such assets.
If we were successfully sued for product liability, we could face substantial liabilities that exceed our resources.
Our business exposes us to potential liability risks inherent in the testing, marketing and processing of molecular diagnostic products, including possible misdiagnoses. Although we are insured against such risks in amounts that we believe to be commercially reasonable, our present professional and product liability insurance may be inadequate. A successful product liability claim in excess of our insurance coverage could have a material adverse effect on our business. Any successful product liability claim may prevent us from obtaining adequate product liability insurance in the future on commercially desirable or reasonable terms. An inability to obtain sufficient insurance coverage at an acceptable cost or otherwise to protect against potential product liability claims could prevent or inhibit the commercialization of our products.
We are dependent on our information technology and telecommunications systems, and any failure of these systems could harm our business.
We depend on information technology ("IT") and telecommunications systems for significant aspects of our business. These IT and telecommunications systems support a variety of functions, including sample processing, tracking, quality control, customer service and support, billing, research and development activities, and various general and administrative activities. Failures or significant downtime of our IT or telecommunications systems could prevent us from processing samples, providing test results to physicians, billing payors, addressing patient or physician inquiries, conducting research and development activities and conducting general and administrative elements of our business. Any disruption or loss of IT or telecommunications systems on which critical aspects of our operations depend could have an adverse effect on our business, financial condition and results of operations.
28
Security breaches, loss of data and other disruptions, including from cyberattacks, could compromise sensitive information related to our business, prevent us from accessing critical information or expose us to liability, which could adversely affect our business and our reputation.
In the ordinary course of our business, we collect and store sensitive data, including legally protected patient health information, credit card information, personally identifiable information about our employees, intellectual property, and proprietary business information. We manage and maintain our applications and data utilizing on-site, remote, or cloud-based systems. These applications and data encompass a wide variety of business-critical information including research and development information, commercial information and business and financial information.
The secure processing, storage, maintenance and transmission of this critical information is vital to our operations and business strategy, and we devote significant resources to protecting such information. Although we take measures to protect sensitive information from unauthorized access or disclosure, our information technology and infrastructure may be vulnerable to attacks by hackers, or viruses, malware, including ransomware, breaches or interruptions due to employee error, malfeasance or other disruptions, or lapses in compliance with privacy and security mandates. Any such malicious cyberattack, virus, breach or interruption could compromise our networks and the information stored there could be accessed by unauthorized parties, publicly disclosed, held for ransom, lost or stolen. We have measures in place that are designed to prevent, and if necessary, to detect and respond to such cybersecurity incidents and breaches of privacy and security mandates. While we have experienced unauthorized accesses to our information technology systems and infrastructure in the past, which may occur again in the future, our security measures have been able to detect, respond to and prevent any material adverse effect to our information systems and business operations from such breaches. However, in the future, any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, such as HIPAA, government enforcement actions and civil or even criminal penalties. Unauthorized access, loss or dissemination could also disrupt our operations, including our ability to process samples, provide test results, bill payors or patients, provide customer support services, conduct research and development activities, process and prepare company financial information, and manage various general and administrative aspects of our business, and may damage our reputation, any of which could adversely affect our business, financial condition and results of operations.
State privacy and data security laws are becoming more stringent. For example, California recently adopted the California Consumer Privacy Act of 2018 (“CCPA”), which was effective in January 2020. The CCPA establishes a new privacy framework for covered businesses by creating an expanded definition of personal information, establishing new data privacy rights for consumers in the State of California, imposing special rules on the collection of consumer data from minors, and creating a new and potentially severe statutory damages framework for violations of the CCPA and for businesses that fail to implement reasonable security procedures and practices to prevent data breaches. In addition to the CCPA, other states are introducing similar legislation which will impact compliance obligations and increase complexity and cost of compliance.
In May 2016, the European Union (“EU”) formally adopted the GDPR, which applies to all EU member states from May 25, 2018. The GDPR introduced stringent new data protection requirements for business activities in the European Union and substantial fines for breaches of the EU data protection rules. The GDPR has increased our responsibility and liability in relation to personal data that we process, and we may be required to put in place additional procedures to ensure compliance with the new EU data protection rules. The GDPR is a complex law with still evolving regulatory guidance, including with respect to how the GDPR should be applied in the context of clinical studies or other transactions from which we may gain access to personal data. Furthermore, many of the countries within the European Union are still in the process of drafting supplementary data protection legislation in key fields where the GDPR allows for national variation, including the fields of clinical study and other health-related information. These national variations may raise our costs of compliance and result in greater potential legal risks.
We may be adversely impacted if we are unable to successfully implement new systems or unable to adapt systems to our change in fiscal year-end.
IT systems are an important part of our business operations. We are in the midst of a multi-year transformation project to achieve better analytics and process efficiencies through the use of Oracle Fusion Cloud Services System (“Oracle Fusion”). This project is expected to improve the efficiency and effectiveness of certain financial and business transaction processes and the underlying systems environment. During the quarter ended September 30, 2020, we completed the implementation of certain modules used in the financial statement close process and management reporting. Additional integrations are expected to take place over the next year. An implementation of this nature is a major undertaking from a financial, management and personnel perspective. The implementation of Oracle Fusion may prove to be more difficult, costly, or time consuming than expected, and there can be no assurance that this system will be beneficial to the extent anticipated.
29
In addition, we changed our fiscal year end from a fiscal year ending on the last day of June of each year to a calendar fiscal year ending on the last day of December each year, effective January 1, 2021, which will require certain modifications to our systems used for accounting and management reporting. If the systems are not appropriately configured for the change in fiscal year-end it could have a material adverse effect on our results of operations and financial condition.
We have identified a material weakness in our internal control over accounting for intercompany transactions, foreign currency exchanges and foreign currency translation related to our international subsidiaries and such weakness led to a conclusion that our internal control over financial reporting and disclosure controls and procedures were not effective as of December 31, 2020. Our ability to remediate the material weakness, our discovery of additional weaknesses, and our inability to achieve and maintain effective disclosure controls and procedures and internal control over financial reporting, could adversely affect our results of operations, our stock price and investor confidence in our company.
Section 404 of the Sarbanes-Oxley Act of 2002 requires that companies evaluate and report on the effectiveness of their internal control over financial reporting. In addition, we engaged our independent registered public accounting firm to report on its evaluation of those controls. As disclosed in more detail under Item 9A, “Controls and Procedures” below, we have identified a material weakness as of December 31, 2020 in our internal control over accounting for intercompany transactions, foreign currency exchanges and foreign currency translation related to our international subsidiaries. Due to the material weakness in our internal control over financial reporting, we have also concluded our disclosure controls and procedures were not effective as of December 31, 2020.
Failure to have effective internal control over financial reporting and disclosure controls and procedures could impair our ability to produce accurate financial statements on a timely basis and could lead to a restatement of our financial statements. For example, the identified material weakness resulted in immaterial corrections to intercompany accounts, as well as foreign currency exchange and translation gains and losses, in our consolidated financial statements for the transition period ended December 31, 2020 and caused a difference between the financial statements we reported in the press release we issued on February 23, 2021 and furnished to the SEC with our Current Report on Form 8-K on the same date and reported in this transition report. Management, however, has concluded that the material weakness did not result in any misstatements that are material to our consolidated financial statements for any of the periods presented. If, as a result of the ineffectiveness of our internal control over financial reporting and disclosure controls and procedures, we cannot provide reliable financial statements, our business decision processes may be adversely affected, our business and results of operations could be harmed, investors could lose confidence in our reported financial information and our ability to obtain additional financing, or additional financing on favorable terms, could be adversely affected. In addition, failure to maintain effective internal control over financial reporting could result in investigations or sanctions by regulatory authorities.
Our management has taken immediate action to begin remediating the material weaknesses, however, certain remedial actions have not started or have only recently been undertaken, and while we expect to continue to implement our remediation plans throughout the fiscal year ended December 31, 2021, we cannot be certain as to when remediation will be fully completed. Additional details regarding the initial remediation efforts are disclosed in more detail under Item 9A, “Controls and Procedures” below. In addition, we may in the future identify additional internal control deficiencies that could rise to the level of a material weakness or uncover other errors in financial reporting. During the course of our evaluation, we may identify areas requiring improvement and may be required to design additional enhanced processes and controls to address issues identified through this review. In addition, there can be no assurance that such remediation efforts will be successful, that our internal control over financial reporting will be effective as a result of these efforts or that any such future deficiencies identified may not be material weaknesses that would be required to be reported in future periods. In addition, we cannot assure you that our independent registered public accounting firm will be able to attest that such internal controls are effective when they are required to do so.
If we fail to remediate the material weakness and maintain effective disclosure controls and procedures or internal control over financial reporting, we may not be able to rely on the integrity of our financial results, which could result in inaccurate or late reporting of our financial results, as well as delays or the inability to meet our reporting obligations or to comply with SEC rules and regulations. Any of these could result in delisting actions by the Nasdaq Stock Market, investigation and sanctions by regulatory authorities, stockholder investigations and lawsuits, and could adversely affect our business and the trading price of our common stock.
30
Our business involves environmental risks that may result in liability for us.
In connection with our research and development activities, we are subject to federal, state and local laws, rules, regulations and policies governing the use, generation, manufacture, storage, air emission, effluent discharge, handling and disposal of certain materials, biological specimens, chemicals and wastes. Although we believe that we have complied with the applicable laws, regulations and policies in all material respects and have not been required to correct any material noncompliance, we may be required to incur significant costs to comply with environmental and health and safety regulations in the future. Although we believe that our safety procedures for handling and disposing of controlled materials comply with the standards prescribed by state and federal regulations, accidental contamination or injury from these materials may occur. In the event of such an occurrence, we could be held liable for any damages that result and any such liability could exceed our resources.
Changes in health care policy could increase our costs, decrease our revenues and impact sales of and reimbursement for our tests.
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, collectively called the ACA, became law. This law substantially changed the way health care is financed by both government and private third-party payors and continues to significantly impact our business and operations in ways we cannot currently predict. Since its enactment, there have been judicial and Congressional challenges to certain aspects of the ACA. Both Congress and former President Trump expressed their intention to repeal or repeal and replace the ACA, and as a result, certain sections of the ACA have not been fully implemented or were effectively repealed. On November 10, 2020, the U.S. Supreme Court upheld the ACA in a 6-3 ruling. Additionally, the new U.S. presidential administration, led by President Biden, are active proponents of the ACA. The uncertainty around the future of the ACA, and in particular the impact to reimbursement levels and the number of insured individuals, may lead to delay in the purchasing decisions of our customers, which may in turn negatively impact our product sales. Further, if reimbursement levels are inadequate, our business and results of operations could be adversely affected.
In addition to the ACA, there will continue to be proposals by legislators at both the federal and state levels, regulators and private third-party payors to reduce costs while expanding individual healthcare benefits. Certain of these changes could impose additional limitations on the prices we will be able to charge for our tests or the amounts of reimbursement available for our tests from governmental agencies or private third-party payors. Any future changes to legal or regulatory requirements or new cost containment initiatives could have a materially adverse effect on our business, financial condition, results of operation, and cash flows.
We face risks associated with currency exchange rate fluctuations, which could adversely affect our operating results.
We receive a portion of our revenues and pay a portion of our expenses in currencies other than the United States dollar, such as the Euro, the Swiss franc, the British pound, the Australian dollar, the Japanese yen, and the Canadian dollar. As a result, we are at risk for exchange rate fluctuations between such foreign currencies and the United States dollar, which could affect the results of our operations. If the U.S. dollar strengthens against foreign currencies, the translation of these foreign currency denominated transactions will result in decreased revenues and operating expenses. We may not be able to offset adverse foreign currency impact with increased revenues. We do not currently utilize hedging strategies to mitigate foreign currency risk and even if we were to implement hedging strategies to mitigate foreign currency risk, these strategies might not eliminate our exposure to foreign exchange rate fluctuations and would involve costs and risks of their own, such as ongoing management time and expertise, external costs to implement the strategies and potential accounting implications.
Risks Related to Commercialization of Our Tests, Our Services and Test Candidates
Our pharmaceutical testing services customers may reduce the amount of testing they conduct through us.
If there is a change in the regulatory environment or intellectual property law, or our pharmaceutical testing services customers consolidate, our customers may divert resources from testing, resulting in a reduced demand for our laboratory testing services. Alternatively, customers may decide to perform their own laboratory testing services in-house.
31
Our molecular diagnostic and companion diagnostic tests in development may never achieve significant commercial market acceptance.
We may not succeed in achieving significant commercial market acceptance of our diagnostic test and clinical service offerings that we have launched in recent years or are currently developing. Our ability to successfully develop and commercialize our current molecular diagnostic and companion diagnostic tests, as well as any future molecular diagnostic and companion diagnostic tests that we may develop, will depend on several factors, including:
•our ability to convince the medical community of the clinical utility of our tests and their potential advantages over existing tests;
•our ability to collaborate with biotechnology and pharmaceutical companies to develop and commercialize companion diagnostic tests for their therapeutic drugs and drug candidates;
•the agreement by third-party payors to reimburse our tests, the scope and extent of which will affect patients’ willingness or ability to pay for our tests and will likely heavily influence physicians’ decisions to recommend our tests; and
•the willingness of physicians to utilize our tests, which can be difficult to interpret. This difficulty is caused by the ability of our tests to predict only as to a probability, not certainty, that a tested individual will develop, have the disease, benefit from a particular therapy or has an aggressive form of the disease that the test is intended to predict.
These factors present obstacles to commercial acceptance of our tests, which we would have to spend substantial time and money to overcome, if we can do so at all. Our inability to successfully do so would harm our business.
If we do not compete effectively with scientific and commercial competitors, we may not be able to successfully commercialize our tests.
The clinical laboratory and genetics testing fields are intense and highly competitive. Tests that are developed are characterized by rapid technological change. Our competitors in the United States and abroad are numerous and include, among others, major diagnostic companies, reference laboratories, molecular diagnostic firms, universities and other research institutions. Some of our potential competitors have considerably greater financial, technical, marketing and other resources than we do, which may allow these competitors to discover important genes and determine their function before we do. We could be adversely affected if we do not discover genes, proteins or biomarkers and characterize their function, develop molecular diagnostic and pharmaceutical and clinical services based on these discoveries, obtain required regulatory and other approvals and launch these tests and their related services before our competitors. We also expect to encounter significant competition with respect to any molecular diagnostic and companion diagnostic tests that we may develop or commercialize. Those companies that bring to market new molecular diagnostic and companion tests before we do may achieve a significant competitive advantage in marketing and commercializing their tests. We may not be able to develop additional molecular diagnostic tests successfully and we or our licensors may not obtain or enforce patents covering these tests that provide protection against our competitors. Moreover, our competitors may succeed in developing molecular diagnostic and companion diagnostic tests that circumvent our technologies or tests. Furthermore, our competitors may succeed in developing technologies or tests that are more effective or less costly than those developed by us or that would render our technologies or tests less competitive or obsolete. We expect competition to intensify in the fields in which we are involved as technical advances in these fields occur and become more widely known and changes in intellectual property laws generate challenges to our intellectual property position.
Our international business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States.
As part of our business strategy, we operate in international markets. Though we recently narrowed our international operations, we have established active sales operations in Germany, France, and Japan; production operations in Germany; and international headquarters in Switzerland. We may establish additional operations or acquire additional properties outside the United States in order to advance our international sales doing business internationally involves a number of risks, including:
•failure by us to obtain regulatory approvals or adequate reimbursement for the use of our tests in various countries;
•ineffective marketing campaigns leading to failure in establishing a viable, profitable, and sustainable presence in our international markets;
•difficulty in staffing and managing foreign operations;
•managing multiple payor reimbursement and self-pay systems;
•logistics and regulations associated with shipping patient samples, including infrastructure conditions and transportation delays;
•limits in our ability to penetrate international markets if we are not able to process tests locally;
32
•financial risks, such as longer payment cycles, difficulty collecting accounts receivable and exposure to foreign currency exchange rate fluctuations;
•political and economic instability, including wars, terrorism, and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions;
•multiple, conflicting and changing laws and regulations such as tax laws, export and import restrictions, employment laws, data and privacy laws such as the EU GDPR, regulatory requirements and other governmental approvals, permits and licenses; and
•regulatory and compliance risks that relate to maintaining accurate information and control over sales and distributors’ activities that may fall within the purview of the U.S. Foreign Corrupt Practice Act, UK Bribery Act, anti-boycott laws and other anti-corruption laws.
Any of these factors could significantly harm our international operations and, consequently, our revenues and results of operations. In addition, any failure to comply with applicable legal and regulatory obligations could impact us in a variety of ways that include, but are not limited to, significant criminal, civil and administrative penalties, including imprisonment of individuals, fines and penalties, denial of export privileges, seizure of shipments, and restrictions on certain business activities. Also, the failure to comply with applicable legal and regulatory obligations could result in the disruption of our distribution and sales activities.
Our international operations could be affected by changes in laws, trade regulations, labor and employment regulations, and procedures and actions affecting approval, production, pricing, reimbursement and marketing of tests, as well as by inter-governmental disputes. Any of these changes could adversely affect our business. Our success internationally will depend, in part, on our ability to develop and implement policies and strategies that are effective in anticipating and managing these and other risks in the countries in which we do business. Failure to manage these and other risks may have a material adverse effect on our operations in any particular country and on our business as a whole.
Foreign governments may impose reimbursement standards, which may adversely affect our future profitability.
We market our tests in foreign jurisdictions and as such may be subject to rules and regulations in those jurisdictions relating to our testing. In some foreign countries, the reimbursement of diagnostic tests is subject to governmental control. In these countries, reimbursement negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a test candidate. If reimbursement of our future tests is unavailable or limited in scope or amount, or if reimbursement rates are set at unsatisfactory levels, we may be unable to achieve or sustain profitability.
International data protection laws and regulations may restrict our activities and increase our costs.
International data protection laws and regulations may affect our collection, use, storage, and transfer of information obtained outside of the United States. In particular, GDPR requires us to meet new and more stringent requirements regarding the handling of personal data about European Union residents. Failure to meet GDPR requirements could result in penalties of up to 4% of our worldwide revenue. The GDPR is a complex law and the regulatory guidance is still evolving. Furthermore, many of the countries within the European Union are still in the process of drafting supplementary data protection legislation in key fields where the GDPR allows for national variation, including the fields of clinical study and other health-related information. These variations in European data protection laws may raise our costs of compliance and result in greater legal risks. Failure to comply with data protection laws and regulations could result in government enforcement actions, which may involve civil and criminal penalties, private litigation and/or adverse publicity and could negatively affect our operating results and business. Claims that we have violated individuals’ privacy rights or breached our contractual obligations, even if we are not found liable, could be expensive and time-consuming to defend and could result in adverse publicity that could harm our business.
33
We rely on a single laboratory facility to process each of our molecular diagnostic tests in the United States and Europe and a single laboratory facility to perform our pharmaceutical and clinical services. Failure to maintain the operations of these laboratories in compliance with applicable regulations would seriously harm our business.
We rely on a CLIA-certified facility in Salt Lake City, Utah to perform most of our molecular diagnostic tests; a CLIA-certified laboratory in South San Francisco, California to perform our Foresight and Prequel tests; a single laboratory facility in Cologne, Germany to perform and produce our EndoPredict test kits; a CLIA-certified laboratory in Mason, Ohio to perform our GeneSight test; and a CLIA-certified laboratory facility in Austin, Texas to perform our pharmaceutical and clinical testing services. These facilities and certain pieces of laboratory equipment would be difficult to replace and may require significant replacement lead-time. In the event any of our clinical testing facilities were to lose its CLIA certification or other required certifications or licenses or were affected by a pandemic or man-made or natural disaster, we would be unable to continue our molecular diagnostic and pharmaceutical and clinical services business at current levels to meet customer demands for a significant period of time. Although we maintain insurance on these facilities, including business interruption insurance, it may not be adequate to protect us from all potential losses if these facilities were damaged or destroyed. In addition, any interruption in our molecular diagnostic or pharmaceutical and clinical services business would result in a loss of goodwill, including damage to our reputation. If our molecular diagnostic or pharmaceutical and clinical services business were interrupted, it would seriously harm our business.
We depend on a limited number of third parties for some of our supplies of equipment and reagents. If these supplies become unavailable or are disrupted, including as a result of COVID-19 and responses to it, then we may not be able to successfully perform our research or operate our business on a timely basis or at all.
We currently rely on a small number of suppliers to provide our gene sequencing equipment, content enrichment equipment, multiplex protein analysis equipment, robots, and specialty reagents and laboratory supplies required in connection with our testing and research. We believe that currently there are limited alternative suppliers of these equipment, robots, and reagents. The equipment, robots, or the reagents may not remain available in commercial quantities at acceptable costs. If we are unable to obtain when needed additional or alternative equipment, robots, or an adequate supply of reagents or other ingredients at commercially reasonable rates, our ability to continue to identify genes and perform molecular diagnostic testing and pharmaceutical and clinical services would be adversely affected.
We have experienced and may continue to experience a shortage of certain laboratory supplies and equipment, and we may experience a suspension of services from other laboratories or third parties as a result of COVID‑19 and ongoing responses to it. Political, administrative, legislative, legal or regulatory actions in response to COVID‑19, including the possible use of the Defense Production Act in the United States to compel manufacturers to prioritize other products or customers over us, could create additional supply shortages, disruptions or other uncertainties affecting our research and business.
If our current research collaborators or scientific advisors terminate their relationships with us or develop relationships with a competitor, our ability to discover genes, proteins, and biomarkers, and to validate and commercialize molecular diagnostic and companion diagnostic tests could be adversely affected.
We have relationships with research collaborators at academic and other institutions who conduct research at our request. These research collaborators are not our employees. As a result, we have limited control over their activities and, except as otherwise required by our collaboration agreements, can expect only limited amounts of their time to be dedicated to our activities. Our ability to discover genes, proteins, and biomarkers involved in human disease and validate and commercialize molecular diagnostic and companion diagnostic tests will depend in part on the continuation of these collaborations. If any of these collaborations are terminated, we may not be able to enter into other acceptable collaborations. In addition, our existing collaborations may not be successful.
Our research collaborators and scientific advisors may have relationships with other commercial entities, some of which could compete with us. Our research collaborators and scientific advisors sign agreements which provide for the confidentiality of our proprietary information. We may not, however, be able to maintain the confidentiality of our technology and other confidential information related to all collaborations. The dissemination of our confidential information could have a material adverse effect on our business.
34
If we fail to retain our key personnel and hire, train and retain qualified employees and consultants, we may not be able to successfully continue our business.
Because of the specialized scientific nature of our business, we are highly dependent upon our ability to attract and retain qualified management, scientific and technical personnel. We are currently recruiting additional qualified management, scientific and technical personnel. Competition for such personnel is intense. Loss of the services of or failure to recruit additional key management, scientific and technical personnel would adversely affect our research and development programs and molecular diagnostic and pharmaceutical and clinical services business and may have a material adverse effect on our business as a whole.
Our agreements with our employees generally provide for employment that can be terminated by either party without cause at any time, subject to specified notice requirements. Further, the non-competition provision to which each employee is subject expires for certain key employees on the applicable date of termination of employment.
Risks Related to Our Intellectual Property
If we are not able to protect our proprietary technology, others could compete against us more directly, which would harm our business.
As of December 31, 2020, our patent portfolio included issued patents owned or licensed by us and numerous patent applications in the United States and other countries with claims protecting our intellectual property rights. Our commercial success will depend, in part, on our ability to obtain additional patents and licenses and protect our existing patent position, both in the United States and in other countries, for compositions, processes, methods and other inventions that we believe are patentable. Our ability to preserve our trade secrets, proprietary data bases and other intellectual property is also important to our long-term success. If our intellectual property is not adequately protected, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to maintain profitability. Patents may also issue to third parties which could interfere with our ability to bring our molecular diagnostic tests to market. The laws of some foreign countries do not protect our proprietary rights to the same extent as U.S. laws, and we may encounter significant problems in protecting our proprietary rights in these countries.
The patent positions of diagnostic companies, including our patent position, are generally highly uncertain and involve complex legal and factual questions, and, therefore, any patents issued to us may be challenged, deemed unenforceable, invalidated or circumvented. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies and any future tests are covered by valid and enforceable patents or are effectively maintained as trade secrets. Our patent applications may never issue as patents, and the claims of any issued patents may not afford meaningful protection for our technology or tests. In addition, any patents issued to us or our licensors may be challenged, and subsequently narrowed, invalidated or circumvented.
Where necessary, we may initiate litigation to enforce our patent or other intellectual property rights. Any such litigation may require us to spend a substantial amount of time and money and could distract management from our day-to-day operations. Moreover, there is no assurance that we will be successful in any such litigation.
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
•we or our licensors were the first to make the inventions covered by each of our patent applications;
•we or our licensors were the first to file patent applications for these inventions;
•others will not independently develop similar or alternative technologies or duplicate any of our technologies;
•any of our or our licensors’ patent applications will result in issued patents;
•any of our or our licensors’ patents will be valid or enforceable;
•any patents issued to us or our licensors and collaborators will provide a basis for commercially viable tests, will provide us with any competitive advantages or will not be challenged by third parties;
•we will develop additional proprietary technologies or tests that are patentable;
•the patents of others will not have an adverse effect on our business; or
•our patents or patents that we license from others will survive legal challenges and remain valid and enforceable.
35
If a third party files a patent application with claims to subject matter we have invented, the United States Patent and Trademark Office (“USPTO”) may declare interference between competing patent applications. If an interference is declared, we may not prevail in the interference. If the other party prevails in the interference, we may be precluded from commercializing services or tests based on the invention or may be required to seek a license. A license may not be available to us on commercially acceptable terms, if at all. For example, in January 2020 the Patent Trial and Appeal Board (the “PTAB”) of the USPTO declared patent Interference No. 106,122 between U.S. Patent No. 9,200,324 controlled by Myriad (under the license agreement with OMRF) relating to the Vectra test and U.S. Application No. 15/363,991 owned by Meso Scale Technologies, LLC. On February 24, 2021 the PTAB issued a decision and judgment denying Meso Scale's motion for a finding of priority and inventorship in their favor and refusing Meso Scale's claims in U.S. Application No. 15/363,991. The PTAB's judgment may be appealed.
We also rely upon unpatented proprietary technologies and databases. Although we require employees, consultants and collaborators to sign confidentiality agreements, we may not be able to adequately protect our rights in such unpatented proprietary technologies and databases, which could have a material adverse effect on our business. For example, others may independently develop substantially equivalent proprietary information or techniques or otherwise gain access to our proprietary technologies or disclose our technologies to our competitors.
If we were sued for patent infringement by third parties, we might incur significant costs and delays in test introduction.
Our tests may also conflict with patents that have been or may be granted to others. Our industry includes many organizations that have or are seeking to discern biomarkers and develop genomic, proteomic and other technologies. To the extent any patents are issued or have been issued to those organizations, the risk increases that the sale of our molecular diagnostic and companion diagnostic tests currently being marketed or under development may give rise to claims of patent infringement. Others may have filed and in the future are likely to file patent applications covering biomarkers that are similar or identical to our tests. Any of these patent applications may have priority over our patent applications and these entities or persons could bring legal proceedings against us seeking damages or seeking to enjoin us from testing or marketing our tests. Patent litigation is costly, and even if we prevail, the cost of such litigation could have a material adverse effect on us. If the other parties in any such actions are successful, in addition to any liability for damages, we could be required to cease the infringing activity or obtain a license. Any license required may not be available to us on commercially acceptable terms, if at all. Our failure to obtain a license to any technology that we may require to commercialize our tests could have a material adverse effect on our business.
We believe that there may be significant litigation in the industry regarding patent and other intellectual property rights. If we become involved in this litigation, it could consume a substantial portion of our managerial and financial resources.
We may be unable to adequately prevent disclosure of trade secrets, proprietary databases, and other proprietary information.
We rely on trade secrets to protect our proprietary technologies and databases, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers and others to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy if unauthorized disclosure of confidential information occurs. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive position.
If we fail to comply with our obligations under license or technology agreements with third parties, we could lose license rights that are critical to our business.
We license intellectual property that is important to our business, including licenses underlying the technology in our molecular diagnostic and pharmaceutical and clinical services, and in the future, we may enter into additional agreements that provide us with licenses to valuable intellectual property or technology. These licenses impose various royalty payments, milestones, and other obligations on us. If we fail to comply with any of these obligations, the licensor may have the right to terminate the license. Termination by the licensor would cause us to lose valuable rights, and could prevent us from distributing our current tests, or inhibit our ability to commercialize future test candidates. Our business would suffer if any current or future licenses terminate, if the licensors fail to abide by the terms of the license, if the licensors fail to prevent infringement by third parties, if the licensed patents or other rights are found to be invalid or unenforceable, or if we are unable to enter into necessary licenses on acceptable terms.
36
We may be subject to claims that we or our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
As is commonplace in our industry, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Risks Related to Government Regulation
If we fail to comply with the complex federal, state, local and foreign laws and regulations that apply to our business, we could suffer severe consequences that could materially and adversely affect our operating results and financial condition.
Our operations are subject to extensive federal, state, local and foreign laws and regulations, all of which are subject to change. These laws and regulations currently include, among other things:
•CLIA, which requires that laboratories obtain certification from the federal government, and state licensure laws;
•FDA laws and regulations that apply to medical devices such as our in vitro diagnostics;
•HIPAA, which imposes comprehensive federal standards with respect to the privacy and security of protected health information and requirements for the use of certain standardized electronic transactions; amendments to HIPAA under HITECH, which strengthened and expanded HIPAA privacy and security compliance requirements, increased penalties for violators, extended enforcement authority to state attorneys general and imposed requirements for breach notification;
•state laws regulating genetic testing and protecting the privacy of genetic test results, as well as state laws protecting the privacy and security of health information and personal data and mandating reporting of breaches to affected individuals and state regulators;
•the federal Anti-Kickback Statute, which prohibits knowingly and willfully offering, paying, soliciting, receiving, or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for, or recommending of an item or service that is reimbursable, in whole or in part, by a federal health care program;
•the federal False Claims Act, which imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to the federal government;
•the federal Civil Monetary Penalties Law, which prohibits, among other things, the offering or transfer of remuneration to a Medicare or state health care program beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner, or supplier of services reimbursable by Medicare or a state health care program, unless an exception applies;
•other federal and state fraud and abuse laws, such as anti-kickback laws, prohibitions on self-referral, and false claims acts, which may extend to services reimbursable by any third-party payor, including private insurers;
•the federal Physician Payments Sunshine Act, which requires medical device manufactures to track and report to the federal government certain payments and other transfers of value made to physicians and teaching hospitals and ownership or investment interests held by physicians and their immediate family members;
•Section 216 of the federal Protecting Access to Medicare Act of 2014 (“PAMA”), which requires the Centers for Medicare & Medicaid Services to set Medicare rates for clinical laboratory testing based on private payor data reported by applicable laboratories;
•state laws that impose reporting and other compliance-related requirements; and
•similar foreign laws and regulations that apply to us in the countries in which we operate.
As a clinical laboratory, our business practices may face heightened scrutiny from government enforcement agencies such as the Department of Justice, the OIG, and CMS. The OIG has issued fraud alerts in recent years that identify certain arrangements between clinical laboratories and referring physicians as implicating the Anti-Kickback Statute. The OIG has stated that it is particularly concerned about these types of arrangements because the choice of laboratory, as well as the decision to order laboratory tests, typically are made or strongly influenced by the physician, with little or no input from the patient. Moreover, the provision of payments or other items of value by a clinical laboratory to a referral source could be prohibited under the federal self-referral prohibition, commonly known as the Stark Law or the Physician Self-Referral Law, unless the arrangement meets all criteria of an applicable exception. The government has actively enforced these laws against clinical laboratories in recent years.
37
These laws and regulations are complex and are subject to interpretation by the courts and by government agencies. Our failure to comply could lead to civil or criminal penalties, exclusion from participation in state and federal health care programs, or prohibitions or restrictions on our laboratories’ ability to provide or receive payment for our services. We believe that we are in material compliance with all statutory and regulatory requirements, but there is a risk that one or more government agencies could take a contrary position, or that a private party could file suit under the qui tam provisions of the federal False Claims Act or a similar state law. Such occurrences, regardless of their outcome, could damage our reputation and adversely affect important business relationships with third parties, including managed care organizations, and other private third-party payors.
The growth of our business and our expansion outside of the United States may increase the potential of violating similar foreign laws or our internal policies and procedures. The risk of us being found in violation of these or other laws and regulations is further increased by the fact that many have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Any of the foregoing consequences could seriously harm our business and our financial results.
FDA regulation of our industry generally or our tests specifically could be disruptive to our business.
As described further below, the FDA has long claimed authority to regulate laboratory-developed tests but has exercised its “enforcement discretion” to limit enforcement of in vitro diagnostic regulatory requirements on this category of products. More recently, the FDA has appeared to increase its attention to the marketing of pharmacogenetic tests. For example, in late 2018, the FDA issued a safety communication regarding “genetic tests that claim results can be used to help physicians identify which antidepressant medication would have increased effectiveness or side effects compared to other antidepressant medications.” This safety communication explained that the FDA had reached out to several firms marketing such pharmacogenetic tests where the FDA believed the relationship between genetic variations and a medication’s effects had not been established, including a warning letter to Inova Genomics Laboratory.
In early 2019, we provided the FDA with clinical evidence and other information to support our GeneSight Psychotropic test. Later that year, the FDA requested changes to the GeneSight test offering. Although we disagreed that changes to the test were required, we submitted a proposal regarding the reporting of GeneSight test results to healthcare providers that we believed addressed the FDA’s principal concerns. We believe this approach should not affect the benefits that we believe are provided by the GeneSight test.
Since submitting our proposal to the FDA, we have continued to engage with our trade association in their efforts to defend the offering of pharmacogenomic tests as LDTs and to monitor broader developments across the stakeholder community. In response to public letters from the national laboratory trade association and patient groups, on February 20, 2020, the FDA announced a new “collaboration between FDA’s Center for Devices and Radiological Health and Center for Drug Evaluation and Research intended to provide the agency’s view of the state of the current science in pharmacogenetics.” Although the announcement again asserted that some of these test offerings may be potentially dangerous, the agency also acknowledged that pharmacogenetic testing “offers promise for informing the selection or dosing of some medications for certain individuals.” In conjunction with the announcement, the FDA also released an updated “Table of Pharmacogenetic Associations,”which lists gene-drug interactions that the agency believes are supported by FDA-approved drug labeling and/or “sufficient scientific evidence based on published literature.” Based on our discussions with the agency over the past year and these recent developments, we have not implemented our earlier proposal or any other changes to the GeneSight Psychotropic test. While we see these developments in 2020 as signaling a positive shift in the FDA’s approach to regulating pharmacogenetic tests, we cannot predict with certainty the outcome of this matter or its timing, or whether the ultimate form of the GeneSight Psychotropic test offering will have an adverse effect on our revenues from the test.
38
Failure to comply with laws and regulations related to submission of claims for our services could result in significant monetary damages and penalties and exclusion from the Medicare and Medicaid programs and corresponding foreign reimbursement programs.
We are subject to laws and regulations governing the submission of claims for payment for our services, such as those relating to: coverage of our services under Medicare, Medicaid and other state, federal and foreign health care programs; the amounts that we may bill for our services; and the party to which we must submit claims. Our failure to comply with applicable laws and regulations could result in our inability to receive payment for our services or in attempts by state and federal healthcare programs, such as Medicare and Medicaid, to recover payments already made. Submission of claims in violation of these laws and regulations can result in recoupment of payments already received, substantial civil monetary penalties, and exclusion from state and federal health care programs, and can subject us to liability under the federal False Claims Act and similar laws. The failure to report and return an overpayment to the Medicare or Medicaid program within 60 days of identifying its existence can give rise to liability under the False Claims Act. Further, a government agency could attempt to hold us liable for causing the improper submission of claims by another entity for services that we performed if we were found to have knowingly participated in the arrangement at issue.
We are currently subject to government investigation(s), the unfavorable outcome of which may have a material adverse effect on our financial condition, results of operations and cash flows.
In June 2016, our wholly-owned subsidiary, Crescendo Bioscience, Inc. (“CBI”), received a subpoena from the Office of Inspector General of the Department of Health and Human Services requesting that CBI produce documents relating to entities that received payment from CBI for the collection and processing of blood specimens for testing, including a named unrelated company, healthcare providers and other third party entities. The Office of Inspector General subsequently requested additional documentation in December 2017. CBI provided to the Office of Inspector General the documents requested. On January 30, 2020, the United States District Court for the Northern District of California unsealed a qui tam complaint, filed on April 16, 2016 against CBI, alleging violations of the Federal and California False Claims Acts and the California Insurance Fraud Prevention Act. On January 22, 2020, after a multi-year investigation into CBI’s and the Company’s alleged conduct, the United States declined to intervene. On January 27, 2020, the State of California likewise filed its notice of declination. The Company was not aware of the complaint until after it was unsealed. On April 16, 2020, CBI filed a motion to dismiss the action with prejudice. On May 23, 2020, the court denied that motion. The Company intends to continue to vigorously defend against this action. We are unable to predict what action, if any, might be taken in the future by the Office of Inspector General or any other regulatory authority as a result of the matters related to this investigation.
The above case may divert management resources and/or cause us to incur substantial costs, and any unfavorable outcome may have a material adverse effect on our financial condition, results or operations and cash flows.
Our business could be harmed by the loss, suspension, or other restriction on a license, certification, or accreditation, or by the imposition of a fine or penalties, under CLIA, its implementing regulations, or other state, federal and foreign laws and regulations affecting licensure or certification, or by future changes in these laws or regulations.
The diagnostic testing industry is subject to extensive laws and regulations, many of which have not been interpreted by the courts. CLIA requires virtually all laboratories to be certified by the federal government and mandates compliance with various operational, personnel, facilities administration, quality and proficiency testing requirements intended to ensure that testing services are accurate, reliable and timely. CLIA certification is also a prerequisite to be eligible to bill state and federal health care programs, as well as many private third-party payors, for laboratory testing services. As a condition of CLIA certification, each of our laboratories is subject to survey and inspection every other year, in addition to being subject to additional random inspections. The biennial survey is conducted by CMS; a CMS agent (typically a state agency); or, if the laboratory holds a CLIA certificate of accreditation, a CMS-approved accreditation organization. Sanctions for failure to comply with CLIA requirements, including proficiency testing violations, may include suspension, revocation, or limitation of a laboratory’s CLIA certificate, which is necessary to conduct business, as well as the imposition of significant fines or criminal penalties. In addition, we are subject to regulation under state laws and regulations governing laboratory licensure. Some states have enacted state licensure laws that are more stringent than CLIA. We are also subject to laws and regulations governing our reference laboratory in Germany. Changes in state or foreign licensure laws that affect our ability to offer and provide diagnostic services across state or foreign country lines could materially and adversely affect our business. In addition, state and foreign requirements for laboratory certification may be costly or difficult to meet and could affect our ability to receive specimens from certain states or foreign countries.
39
Any sanction imposed under CLIA, its implementing regulations, or state or foreign laws or regulations governing licensure, or our failure to renew a CLIA certificate, a state or foreign license, or accreditation, could have a material adverse effect on our business. If the CLIA certificate of any one of our laboratories is revoked, CMS could seek revocation of the CLIA certificates of our other laboratories based on their common ownership or operation, even though they are separately certified.
Changes in the way that the FDA regulates tests performed by laboratories like ours could result in delay or additional expense in offering our tests and tests that we may develop in the future.
Historically, the FDA has exercised enforcement discretion with respect to most LDTs and has generally not required laboratories that furnish LDTs to comply with the agency’s requirements for medical devices (e.g., establishment registration, device listing, quality systems regulations, premarket clearance or premarket approval, and post-market controls). In recent years, however, the FDA publicly announced its intention to regulate certain LDTs and issued two draft guidance documents that set forth a proposed phased-in risk-based regulatory framework that would apply varying levels of FDA oversight to LDTs. However, these guidance documents were not finalized, and the framework was abandoned and replaced by an informal discussion paper reflecting some of the feedback that FDA had received on LDT regulation. The FDA acknowledged that the January 2017 discussion paper does not represent the formal position of the FDA and is not enforceable. Nevertheless, the FDA wanted to share its synthesis of the feedback that it had received in the hope that it might advance public discussion on future LDT oversight. Notwithstanding the discussion paper, the FDA continues to exercise enforcement discretion and may attempt to regulate certain LDTs on a case-by-case basis at any time, which could result in delay or additional expense in offering our tests and tests that we may develop in the future.
In addition to potential enforcement priority changes from the FDA, in December 2018, members of Congress released a discussion draft of a legislation to regulate in vitro clinical tests including LDTs under a shared FDA/CMS framework, and provided opportunities for stakeholders to comment on the proposed legislation. On March 5, 2020, U.S. Representatives Diana DeGette (D-CO) and Dr. Larry Bucshon (R-IN) formally introduced the legislation, called the Verifying Accurate, Leading-edge IVCT Development (VALID) Act. An identical version of the bill was also introduced in the Senate and is sponsored by U.S. Senators Michael Bennet (D-CO) and Richard Burr (R-NC), demonstrating both bicameral and bipartisan support for the effort to overhaul how diagnostic tests are regulated. The VALID Act would codify into law the term “in vitro clinical test” (IVCT) to create a new medical product category separate from medical devices that includes products currently regulated as in vitro diagnostics (IVDs) as well as LDTs. The framework would give the FDA the authority to ensure IVCTs are both analytically and clinically valid. CMS would retain the authority to ensure the quality of operations within laboratories. All LDTs on the market prior to enactment of the legislation would be grandfathered and not subject to the new regulation.
It is unclear whether the VALID Act will be passed by Congress in its current form or signed into law by the President. Until the FDA finalizes its regulatory position regarding LDTs, or the VALID Act or other legislation is passed reforming the federal government’s regulation of LDTs, it is unknown how the FDA may attempt to regulate our tests in the future and what testing and data may be required to support any required clearance or approval of our tests by the agency. If the VALID Act is implemented as drafted it could have an adverse material impact on our results of operations.
Companion and complementary diagnostic tests require FDA approval and we may not be able to secure such approval in a timely manner or at all.
Our companion and complementary diagnostic products, marketing, sales and development activities and manufacturing processes are subject to extensive and rigorous regulation by the FDA pursuant to the Federal Food, Drug, and Cosmetic Act (FDCA), by comparable agencies in foreign countries, and by other regulatory agencies and governing bodies. Under the FDCA, companion diagnostics must receive FDA clearance or approval before they can be commercially marketed in the U.S. The process of obtaining marketing approval or clearance from the FDA or by comparable agencies in foreign countries for new products could:
•take a significant period of time;
•require the expenditure of substantial resources;
•involve rigorous pre-clinical testing, as well as increased post-market surveillance;
•require changes to products; and
•result in limitations on the indicated uses of products.
Although we have successfully achieved FDA approval for some tests (e.g., our BRACAnalysis CDx and myChoice CDx tests), we cannot predict whether or when we will be able to obtain FDA approval for other companion diagnostics that we are developing.
40
If the government and third-party payors fail to provide coverage and adequate payment for our existing and future tests, if any, our revenue and prospects for profitability will be harmed.
In both domestic and foreign markets, sales of our molecular diagnostic tests or any future diagnostic tests will depend in large part, upon the availability of reimbursement from third-party payors. Such third-party payors include state and federal health care programs such as Medicare, managed care providers, private health insurers and other organizations. These third-party payors are increasingly attempting to contain healthcare costs by demanding price discounts or rebates and limiting both coverage regarding which diagnostic tests they will pay for and the amounts that they will pay for existing and new molecular diagnostic tests. We have recently experienced price reductions from CMS for some of our products, including for our GeneSight® psychotropic test subsequent to the July 2020 release of the final pharmacogenomics LCD, and we may experience future price reductions from CMS, managed care organizations, and other third-party payors. The fact that a diagnostic test has been approved for reimbursement in the past, for any particular indication or in any particular jurisdiction, does not guarantee that such a diagnostic test will remain approved for reimbursement, that the reimbursement amount approved for such test will not be reduced in the future, or that similar or additional diagnostic tests will be approved in the future. Moreover, there can be no assurance that any new tests we have launched or may launch will be reimbursed at rates that are comparable to the rates that we historically obtained for our existing product portfolio. As a result, third-party payors may not cover or provide adequate payment for our current or future molecular diagnostic tests to enable us to maintain past levels of revenue or profitability with respect to such tests. Further, third-party reimbursement might not be available to enable us to maintain price levels sufficient to realize an appropriate return on investment in product development. In addition, under PAMA, Medicare reimbursement for any given diagnostic test is based on the weighted-median of the payments made by private payors for such test, rendering private payor payment levels even more significant. As a result, future Medicare payments may fluctuate more often and become subject to the willingness of private payors to recognize the value of diagnostic tests generally and any given test individually. In March 2020, Congress passed the Coronavirus Aid, Relief, and Economic Security Act, which included a provision that delays the next PAMA reporting period for clinical laboratory tests that are not advanced diagnostic tests to January 1, 2022 through March 31, 2022. In addition, the next round of rate cuts will not be implemented until 2022, with tests receiving cuts of up to 15 percent a year from 2022 through 2024. Any declines in average selling prices of our products due to pricing pressures may have an adverse impact on our business, results of operations and financial condition.
U.S. and foreign governments continue to propose and pass legislation designed to reduce the cost of health care. For example, in some foreign markets, the government controls the pricing of many health care products. We expect that there will continue to be federal and state proposals to implement governmental controls or impose health care requirements. In addition, the Medicare program and increasing emphasis on managed care in the United States will continue to put pressure on product pricing. Cost control initiatives could decrease the price that we would receive for any tests in the future, which would limit our revenue and profitability.
Our business could be adversely impacted by our failure or the failure of physicians to comply with any new ICD Code Set.
CMS periodically adopts new coding set for diagnoses, commonly known as ICD code sets. Compliance with ICD is required for all claims with dates of service on or after the effective dates specified when such code sets are adopted. We believe we have fully implemented the current ICD-10-CM code set and expect to be able to implement any future code set, however, our failure to implement and apply this or any new code set could adversely impact our business. In addition, if physicians fail to provide appropriate codes for desired tests, we may not be reimbursed for tests we perform.
Risks Related to Our Common Stock
Our stock price is highly volatile, and our stock may lose all or a significant part of its value.
The market prices for securities of molecular diagnostic companies have been volatile. This volatility has significantly affected the market prices for these securities for reasons frequently unrelated to the operating performance of the specific companies. These broad market fluctuations may adversely affect the market price of our common stock. The market price for our common stock has fluctuated significantly since public trading commenced in October 1995, and it is likely that the market price will continue to fluctuate in the future. In the two years ended December 31, 2020, our stock price has ranged from $9.24 per share to $48.40 per share. In addition, the stock market in general has experienced extreme price and volume fluctuations. Events or factors that may have a significant impact on our business and on the market price of our common stock include the following:
•major market events, such as the market’s reaction to the COVID-19 pandemic generally and its specific impact on the Company;
•failure of any of our recently launched tests and any new test candidates to achieve commercial success;
•failure to sustain revenue growth or margins in our molecular diagnostic business;
41
•changes in the structure of healthcare payment systems and changes in governmental or private insurer reimbursement levels for our molecular diagnostic tests;
•introduction of new commercial tests or technological innovations by competitors;
•termination of the licenses underlying our molecular diagnostic and pharmaceutical and clinical services;
•delays or other problems with operating our laboratory facilities;
•failure of any of our research and development programs;
•changes in intellectual property laws or enforcement of our patents in the United States and foreign countries;
•developments or disputes concerning patents or other proprietary rights involving us directly or otherwise affecting the industry as a whole;
•missing or changing the financial guidance we provide;
•changes in estimates or recommendations by securities analysts relating to our common stock or the securities of our competitors;
•changes in the government regulatory approval process for our existing and new tests;
•failure to meet estimates or recommendations by securities analysts that cover our common stock;
•public concern over our approved tests and any test candidates;
•litigation;
•government and regulatory investigations;
•future sales or anticipated sales of our common stock by us or our stockholders;
•the timing and amount of any repurchases of our common stock;
•general market conditions;
•seasonal slowness in sales, particularly in the quarters ending September 30 and March 31, the effects of which may be difficult to understand during periods of growth;
•general perception of public health, the DNA industry and our products;
•economic, healthcare and diagnostic trends, disasters or crises and other external factors; and
•period-to-period fluctuations in our financial results.
These and other external factors may cause the market price and demand for our common stock to fluctuate substantially, which may limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock. In addition, securities class action litigation such as the current stockholder suit pending against the Company discussed below may affect the market price and demand for our common stock. If any of our other stockholders brought another lawsuit against us, we could incur substantial costs defending the lawsuit regardless of the outcome. Such a lawsuit could also divert the time and attention of our management.
Anti-takeover provisions of Delaware law, provisions in our charter and bylaws and re-adoption of our stockholders’ rights plan, or poison pill, could make a third-party acquisition of us difficult.
Because we are a Delaware corporation, the anti-takeover provisions of Delaware law could make it more difficult for a third party to acquire control of us, even if the change in control would be beneficial to stockholders. We are subject to the provisions of Section 203 of the General Corporation Law of Delaware, which prohibits us from engaging in certain business combinations, unless the business combination is approved in a prescribed manner. In addition, our restated certificate of incorporation and restated bylaws also contain certain provisions that may make a third-party acquisition of us difficult, including:
•a classified board of directors, with three classes of directors each serving a staggered three-year term;
•the ability of the board of directors to issue preferred stock;
•a 70% super-majority stockholder vote to amend our bylaws and certain provisions of our certificate of incorporation; and
•the inability of our stockholders to call a special meeting or act by written consent.
In the past, we implemented a stockholders’ rights plan, also called a poison pill, which could make it uneconomical for a third party to acquire the Company on a hostile basis. Although the plan expired in July 2011, our Board of Directors could adopt a new plan at any time. The provisions in a stockholders’ rights plan, as well as Section 203, may discourage certain types of transactions in which our stockholders might otherwise receive a premium for their shares over the then-current market price, and may limit the ability of our stockholders to approve transactions that they think may be in their best interests.
Item 1B. UNRESOLVED STAFF COMMENTS
None.
42
Item 2. PROPERTIES
Our corporate headquarters and facilities are located in Salt Lake City, Utah. We currently lease a total of approximately 335,000 square feet of building space in Salt Lake City dedicated to research and development, administration and our laboratory that has received federal certification under CLIA. Activities related to our oncology, urology, autoimmune, dermatology and women’s health molecular diagnostic business are performed at this location. The leases on our existing Salt Lake City facilities have terms of five to fifteen years, expiring from 2022 through 2027, and provide for renewal options for up to ten additional years. In addition, in December 2018 we entered into a lease agreement for a building, which is currently under construction and will contain approximately 125,000 square feet of additional office space upon completion. We anticipate completion of the building during the first half of calendar year 2021.
We also lease approximately 93,000 square feet in South San Francisco, California under two leases that expire in April 2025 and September 2025. This space is dedicated to administration, research and development and the CLIA-certified laboratory for our women’s health business.
In addition, we lease approximately 36,000 square feet in Austin, Texas under a lease that expires in June 2025. This space is dedicated to administration, research and development and the CLIA-certified laboratory used for pharmaceutical and clinical services, which are performed at this location.
We also lease 2 spaces in Mason, Ohio, the leases for which will expire in December 2021 and August 2024 respectively, and one in Toronto, Ontario, Canada, which is month to month, with a total square footage for the 3 lease spaces of approximately 35,000.
We also maintain leases for several small office locations, such as our manufacturing facility located in Cologne, Germany. These spaces are generally used for the administration of our international operations.
We believe that our existing facilities and equipment are well maintained and in good working condition. We believe our current facilities and those planned will provide adequate capacity for at least the next two years. We continue to make investments in capital equipment as needed to meet the anticipated demand for our molecular diagnostic tests and our pharmaceutical and clinical services.
43
Item 3. LEGAL PROCEEDINGS
Qui Tam Lawsuit
In June 2016, our wholly-owned subsidiary, Crescendo Bioscience, Inc. (“CBI”), received a subpoena from the Office of Inspector General of the Department of Health and Human Services requesting that CBI produce documents relating to entities that received payment from CBI for the collection and processing of blood specimens for testing, including a named unrelated company, healthcare providers and other third party entities. The Office of Inspector General subsequently requested additional documentation in December 2017. CBI provided to the Office of Inspector General the documents requested. On January 30, 2020, the United States District Court for the Northern District of California unsealed a qui tam complaint, filed on April 16, 2016 against CBI and the Company, alleging violations of the Federal and California False Claims Acts and the California Insurance Fraud Prevention Act. On January 22, 2020, after a multi-year investigation into CBI’s and the Company’s alleged conduct, the United States declined to intervene. On January 27, 2020, the State of California likewise filed its notice of declination. The Company was not aware of the complaint until after it was unsealed. On May 23, 2020, the court denied CBI and the Company’s motion to dismiss. The Company intends to continue to vigorously defend against this action. Due to the nature of this matter and inherent uncertainties, it is not possible to provide an evaluation of the likelihood of an unfavorable outcome or an estimate of the amount or range of potential loss, if any.
Purported Securities Class Action
On September 27, 2019, a purported class action complaint was filed in the United States District Court for the District of Utah, against the Company, its former President and Chief Executive Officer, Mark C. Capone, and its Interim President and Chief Executive Officer, Executive Vice President and Chief Financial Officer, R. Bryan Riggsbee (“Defendants”). On February 21, 2020, the plaintiff filed an amended class action complaint, which added the Company’s Executive Vice President of Clinical Development, Bryan M. Dechairo, as an additional Defendant. This action, captioned In re Myriad Genetics, Inc. Securities Litigation (No. 2:19-cv-00707-DBB), is premised upon allegations that the Defendants made false and misleading statements regarding our business, operations, and acquisitions. The lead plaintiff seeks the payment of damages allegedly sustained by it and the purported class by reason of the allegations set forth in the amended complaint, plus interest, and legal and other costs and fees. The Company intends to vigorously defend against this action. Due to the nature of this matter and inherent uncertainties, it is not possible to provide an evaluation of the likelihood of an unfavorable outcome or an estimate of the amount or range of potential loss, if any.
Other Legal Proceedings
On August 24, 2018, Assurex Health, Inc. was served with an Amended Complaint which had been filed in the Circuit Court of Cook County, Illinois, County Department, Law Division, Civil Action No. 2018 L 004972, by Pipe Trades Services MN Welfare Plan ("Pipe Trades"), as a qui tam relator, on behalf of the State of Illinois, Pipe Trades, and all others similarly situated, purportedly arising from Assurex's alleged violations of the Illinois Insurance Claims Fraud Prevention Act and other causes of action. Pipe Trades seeks certification of a putative class, certification as the purported class representative, and the payment of treble damages allegedly sustained by Pipe Trades and the purported class by reason of the allegations set forth in the amended complaint, plus statutory damages and penalties, plus interest, and legal and other costs and fees. The State of Illinois and Cook County, Illinois, have declined to intervene in the matter. On February 19, 2021, the court granted Assurex's motion to dismiss the complaint, without prejudice and with leave for Pipe Trades to file an amended complaint, for failure to state a claim on which relief can be granted. If Pipe Trades files an amended complaint, we intend to continue to vigorously defend against this action. Due to the nature of this matter and inherent uncertainties, it is not possible to provide an evaluation of the likelihood of an unfavorable outcome or an estimate of the amount or range of potential loss, if any.
Other than as set forth above, we are not a party to any legal proceedings that we believe will have a material impact on our business, financial position or results of operations.
Item 4. MINE SAFETY DISCLOSURES
None.
44
PART II
Item 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is traded on The Nasdaq Global Select Market under the symbol "MYGN."
Stockholders
As of February 24, 2021, there were approximately 105 stockholders of record of our common stock and, according to our estimates, approximately 35,186 beneficial owners of our common stock.
Unregistered Sales of Securities
None.
Issuer Purchases of Equity Securities
Our Board of Directors has previously authorized us to repurchase up to $200.0 million of our outstanding common stock, of which $110.7 million is still available to repurchase as of December 31, 2020. We are authorized to complete the repurchase through open market transactions or through an accelerated share repurchase program, in each case to be executed at management’s discretion based on business and market conditions, stock price, trading restrictions, acquisition activity and other factors. The repurchase program may be suspended or discontinued at any time without prior notice.
No stock repurchases were made under our stock repurchase program during the transition period ended December 31, 2020.
45
Stock Performance Graph
The graph set forth below compares the annual percentage change in our cumulative total stockholder return on our common stock during a period commencing on December 31, 2015 and ending on December 31, 2020 (as measured by dividing (A) the difference between our share price at the end and the beginning of the measurement period; by (B) our share price at the beginning of the measurement period) with the cumulative total return of The Nasdaq Stock Market, Inc. and the Nasdaq Health Care Providers Stock Index during such period. We have not paid any cash dividends on our common stock, and we do not include cash dividends in the representation of our performance. The price of a share of common stock is based upon the closing price per share as quoted on The Nasdaq Global Select Market on the last trading day of the year shown. The graph lines merely connect year-end values and do not reflect fluctuations between those dates. The comparison assumes $100 was invested on December 31, 2015 in our common stock and in each of the foregoing indices. The comparisons shown in the graph below are based upon historical data. We caution that the stock price performance shown in the graph below is not necessarily indicative of, nor is it intended to forecast, the potential future performance of our common stock.
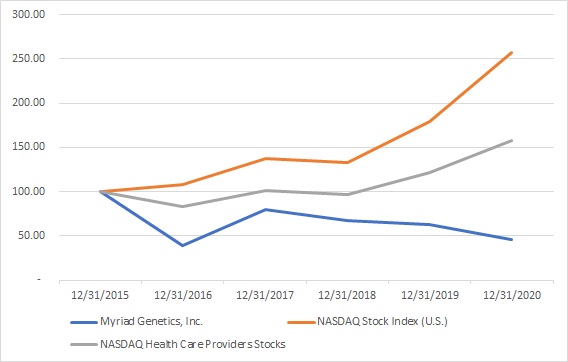
| 12/31/2015 | 12/31/2016 | 12/31/2017 | 12/31/2018 | 12/31/2019 | 12/31/2020 | |||||||||||||||
| Myriad Genetics, Inc. | 100.00 | 38.62 | 79.59 | 67.35 | 63.09 | 45.81 | ||||||||||||||
| NASDAQ Stock Index (U.S.) | 100.00 | 107.50 | 137.86 | 132.51 | 179.19 | 257.38 | ||||||||||||||
| NASDAQ Health Care Providers Stocks | 100.00 | 83.09 | 100.79 | 96.59 | 121.54 | 158.03 | ||||||||||||||
Note: Information used on the graph was obtained from the CRSP Total Return Indexes, a source believed to be reliable, but we are not responsible for any errors or omission in such information.
The performance graph shall not be deemed to be incorporated by reference by means of any general statement incorporating by reference this Form 10-K into any filing under the Securities Act of 1933, as amended or the Securities Exchange Act of 1934, as amended, except to the extent that we specifically incorporate such information by reference, and shall not otherwise be deemed filed under such acts.
46
Item 6. SELECTED CONSOLIDATED FINANCIAL DATA
The following table sets forth our selected consolidated financial data and has been derived from our audited consolidated financial statements. Consolidated balance sheets as of the transition period ended December 31, 2020, and the fiscal years ended June 30, 2020 and 2019, as well as consolidated statements of operations for the transition period ended December 31, 2020 and for the fiscal years ended June 30, 2020, 2019 and 2018 and the reports thereon are included elsewhere in this Transition Report on Form 10-K. The information below should be read in conjunction with our audited consolidated financial statements (and notes thereon) and "Management's Discussion and Analysis of Financial Condition and Results of Operations," included in Item 7.
We adopted Accounting Standards Update No. 2014-09, “Revenue from Contracts with Customers (Topic 606)” using the full retrospective transition method and recast results from 2018 and 2017 including interim periods therein. Results from periods prior to 2017 have not been recast for the adoption of this standard. During the transition period ended December 31, 2020, we identified certain errors that originated in prior periods that we determined to be immaterial and were corrected in the current period. See additional details in Note 1.
| (in millions, except per share amounts) | Transition Period Ended December 31, | Years Ended June 30, | |||||||||||||||||||||||||||||||||
| Consolidated Statement of Operations Data: | 2020 | 2020 | 2019 (a) | 2018 | 2017 (a) | 2016 (a) | |||||||||||||||||||||||||||||
| Total revenue | $ | 299.8 | $ | 638.6 | $ | 851.1 | $ | 743.7 | $ | 728.7 | $ | 740.5 | |||||||||||||||||||||||
| Total costs and expenses | 387.6 | 870.3 | 843.5 | 621.8 | 684.7 | 587.1 | |||||||||||||||||||||||||||||
| Operating income (loss) | (87.8) | (231.7) | 7.6 | 121.9 | 44.0 | 153.4 | |||||||||||||||||||||||||||||
| Total other income (expense) | (6.3) | 8.4 | (7.6) | (1.8) | (7.8) | 2.6 | |||||||||||||||||||||||||||||
| Income (loss) before income taxes | (94.1) | (223.3) | — | 120.1 | 36.2 | 156.0 | |||||||||||||||||||||||||||||
| Income tax provision (benefit) | (41.0) | (23.7) | (4.4) | (13.0) | 19.0 | 38.8 | |||||||||||||||||||||||||||||
| Net income (loss) | (53.1) | (199.6) | 4.4 | 133.1 | 17.2 | 117.2 | |||||||||||||||||||||||||||||
| Net loss attributable to non-controlling interest | — | (0.1) | (0.2) | (0.2) | (0.2) | — | |||||||||||||||||||||||||||||
| Net income (loss) attributable to Myriad Genetics, Inc. stockholders | $ | (53.1) | $ | (199.5) | $ | 4.6 | $ | 133.3 | $ | 17.4 | $ | 117.2 | |||||||||||||||||||||||
| (in millions) | December 31, | June 30, | |||||||||||||||||||||||||||||||||
| Consolidated Balance Sheet Data: | 2020 | 2020 | 2019 | 2018 | 2017 | 2016 | |||||||||||||||||||||||||||||
Cash, cash equivalents and marketable investment securities | $ | 171.7 | $ | 254.8 | $ | 191.8 | $ | 211.3 | $ | 199.2 | $ | 238.9 | |||||||||||||||||||||||
| Working capital | 243.5 | 184.7 | 230.8 | 225.4 | 83.2 | 229.8 | |||||||||||||||||||||||||||||
| Total assets | 1,418.8 | 1,404.6 | 1,562.7 | 1,175.3 | 1,207.9 | 867.2 | |||||||||||||||||||||||||||||
| Noncurrent operating lease liabilities (b) | 50.6 | 56.9 | — | — | — | — | |||||||||||||||||||||||||||||
| Long-term debt | 224.8 | 224.4 | 233.5 | 9.3 | 99.1 | — | |||||||||||||||||||||||||||||
| Stockholders’ equity | 881.0 | 918.2 | 1,088.9 | 966.1 | 767.0 | 739.6 | |||||||||||||||||||||||||||||
(a)We acquired Counsyl, Inc., Assurex Health, Inc., and Sividon Diagnostics GmbH in fiscal years ended June 30, 2019, 2017 and 2016, respectively. As such, the results of each year may not be comparable. See additional details within notes to previously issued financial statements.
(b)Results for the transition period ended December 31, 2020 and for the fiscal year ended June 30, 2020 are presented under ASU 2016-02, Leases. Prior period amounts were not adjusted and continue to be reported under previous lease accounting guidance.
47
Item 7. MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with Part II, ITEM 6 of this Report and the audited Consolidated Financial Statements and accompanying notes thereto included elsewhere in this Report. Unless otherwise noted, all of the financial information in this Report is consolidated financial information for the Company.
Overview
We are a leading precision medicine company acting as a trusted advisor to transform patient lives through molecular diagnostics and are one of the largest specialty molecular diagnostic laboratories in the world. Since our founding in 1992, we have performed tests for approximately five million patients. Through our proprietary technologies, we believe we are positioned to identify important disease genes, the proteins they produce, and the biological pathways in which they are involved to better understand the genetic basis of human disease. We believe that identifying these biomarkers will enable us to develop novel molecular diagnostic tests that can provide important information to solve unmet medical needs.
Our consolidated revenues consist primarily of sales of molecular diagnostic tests and pharmaceutical and clinical services through our wholly-owned subsidiaries. During the transition period ended December 31, 2020, we reported total revenues of $299.8 million, net loss attributable to Myriad Genetics, Inc. stockholders of $53.1 million and basic and diluted loss per share of $0.71.
See Note 15 “Segment and Related Information” in the notes to our Consolidated Financial Statements for information regarding our operating segments.
Industry and Competition
Patients, providers, payors and health systems are looking to apply the power of molecular diagnostics and precision medicine to achieve improved clinical outcomes and lower cost. Key industry trends include:
•Accelerating shifts in consumer engagement, early detection, home-based care models, telemedicine and virtual care;
•Disruption in the way outpatient care is delivered in the wake of the COVID-19 pandemic, coupled with broadened awareness of the vital role of diagnostic testing;
•Expanding access to genetic insights, particularly among underserved populations with increased focus on disparities in healthcare outcomes and access for challenged communities; and
•Growth in personalized medicine and the interest in new partnership models to advance companion diagnostics and serve patients with specific treatments based on their own genetic makeup and biology.
These market trends create new opportunities to position Myriad Genetics, and our products and services, for growth and commercial success through enhanced customer service levels and a stronger alignment of our value proposition with physicians and payors. Our record of innovation will be leveraged not only in research and development and technology, but also in go-to-market approaches and other applications so we can adapt quickly to customer preferences and market dynamics.
Seasonality
We have historically experienced seasonality in our testing business. The quarter ending December 31 is generally strong as we see an increase in volumes from patients who have met their annual insurance deductible. Conversely, in the quarter ending March 31 we see a decrease in volumes due to the annual reset of patient deductibles. Additionally, the volume of testing is negatively impacted by the summer season, which is generally reflected in the quarter ended June 30. Due to the global pandemic, we cannot predict if seasonality will follow the same pattern as in prior years.
48
Components of Consolidated Operations
Revenue
Molecular Diagnostic Testing. Our molecular diagnostic tests are designed to analyze genes and their expression levels to assess an individual’s risk for developing disease later in life, accurately diagnose disease, determine a patient’s likelihood of responding to a particular drug, or disease recurrence, and assess a patient’s risk of disease progression. Provided with this valuable information, physicians may more effectively manage their patients’ healthcare.
Pharmaceutical and Clinical Service. Through Myriad RBM, we provide biomarker discovery, pharmaceutical and clinical services to the pharmaceutical, biotechnology, and medical research industries utilizing our multiplexed immunoassay technology. Our technology enables us to efficiently screen large sets of well-characterized clinical samples from both diseased and non-diseased populations against our extensive menu of biomarkers.
Costs and Expenses
Expenses. Personnel-related costs for each category of Costs and Expenses includes salaries, bonuses, employee benefits costs, employer payroll taxes, and stock-based compensation.
Cost of Molecular Diagnostic Testing. Cost of molecular diagnostic testing consists primarily of costs related to lab supplies, overhead costs, and personnel-related costs.
Cost of Pharmaceutical and Clinical Service. Cost of pharmaceutical and clinical service consists primarily of costs related to lab supplies and personnel-related costs.
Research and Development Expense. Research and development expenses include costs incurred in formulating, improving, validating and creating alternative or modified processes related to and expanding the use of our current molecular diagnostic test offerings and costs incurred for the discovery, development and validation of our pipeline of molecular diagnostic and companion diagnostic candidates.
Change in the Fair Value of Contingent Consideration. Change in the fair value of contingent consideration includes changes in the timing and amount of expected cash payments associated with the contingent consideration related to the Sividon acquisition.
Selling, General and Administrative Expense. Selling, general and administrative expenses include costs associated with managing and growing our businesses domestically and internationally. Selling, general and administrative expenses consist primarily of salaries, commissions and related personnel costs for sales, marketing, customer service, billing and collection, legal, finance and accounting, information technology, human resources, and allocated facilities expenses.
Other Income (Expense). Other income (expense) includes interest income earned on our cash and cash equivalents holdings in short-term interest-bearing accounts; interest expense associated with our debt and amortization of deferred financing costs and original issue discount costs; and foreign currency gains and losses, realized gain or loss on marketable securities, and other nonrecurring income and expenses.
49
Results of Operations
Six Months Ended December 31, 2020 and 2019
Revenue
| Six Months Ended December 31, | Change | % of Total Revenue | |||||||||||||||||||||||||||
| (In millions) | 2020 | 2019 (unaudited) | 2020 | 2020 | 2019 | ||||||||||||||||||||||||
| Molecular diagnostic revenue: | |||||||||||||||||||||||||||||
Hereditary Cancer Testing | $ | 159.3 | $ | 222.2 | $ | (62.9) | 53 | % | 58 | % | |||||||||||||||||||
| GeneSight | 29.8 | 45.2 | (15.4) | 10 | % | 12 | % | ||||||||||||||||||||||
| Prenatal | 37.6 | 39.9 | (2.3) | 13 | % | 10 | % | ||||||||||||||||||||||
| Vectra | 18.0 | 21.4 | (3.4) | 6 | % | 6 | % | ||||||||||||||||||||||
| myChoice CDx | 13.2 | 5.9 | 7.3 | 4 | % | 2 | % | ||||||||||||||||||||||
| Prolaris | 14.8 | 13.3 | 1.5 | 5 | % | 4 | % | ||||||||||||||||||||||
| EndoPredict | 5.9 | 4.8 | 1.1 | 2 | % | 1 | % | ||||||||||||||||||||||
| Other | 1.0 | 0.4 | 0.6 | — | % | — | % | ||||||||||||||||||||||
Total molecular diagnostic revenue | 279.6 | 353.1 | (73.5) | ||||||||||||||||||||||||||
Pharmaceutical and clinical service revenue | 20.2 | 28.3 | (8.1) | 7 | % | 7 | % | ||||||||||||||||||||||
| Total revenue | $ | 299.8 | $ | 381.4 | $ | (81.6) | 100 | % | 100 | % | |||||||||||||||||||
The Company's revenue for the six months ended December 31, 2020 continued to be impacted by factors related to the COVID-19 pandemic, including patients experiencing obstacles to accessing healthcare professionals and deferring elective medical care, which resulted in a decrease in testing volumes compared to the prior year in the majority of the Company's products. In addition, the average expected reimbursement per test decreased due to a variety of factors such as negotiating new contracts with payors and the impact of estimating future refunds across the Company's products.
Hereditary cancer testing revenues for the six months ended December 31, 2020 decreased $62.9 million compared to the six months ended December 31, 2019 due primarily to an approximate 17% decrease in volumes and an approximate 14% decrease in average reimbursement per test. GeneSight revenues for the six months ended December 31, 2020 declined $15.4 million compared to the six months ended December 31, 2019 due primarily to an approximate 23% decrease in volumes and an approximate 14% decrease in average reimbursement per test. Prenatal revenues for the six months ended December 31, 2020 declined $2.3 million compared to the six months ended December 31, 2019 due primarily to a decrease in average reimbursement per test of approximately 15%. Vectra revenues for the six months ended December 31, 2020 declined $3.4 million compared to the six months ended December 31, 2019 primarily due to a decrease in the average reimbursement per test of approximately 13%. These declines were partially offset by an increase in myChoice CDx revenues of $7.3 million due to increased volumes from the test being offered in new markets.
Pharmaceutical and clinical service revenue for the six months ended December 31, 2020 declined $8.1 million compared to the six months ended December 31, 2019, primarily due to the sale of Privatklinik Dr. Robert Schindlbeck GmbH & Co. KG ("the Clinic") in February 2020.
Cost of Sales
| Six Months Ended December 31, | Change | ||||||||||||||||
| (in millions) | 2020 | 2019 (unaudited) | 2020 | ||||||||||||||
| Cost of molecular diagnostic testing | $ | 82.6 | $ | 82.2 | $ | 0.4 | |||||||||||
| Cost of molecular diagnostic testing as a % of revenue | 29.5 | % | 23.3 | % | |||||||||||||
| Cost of pharmaceutical and clinical services | $ | 8.8 | $ | 17.1 | $ | (8.3) | |||||||||||
| Cost of pharmaceutical and clinical services as a % of revenue | 43.6 | % | 60.4 | % | |||||||||||||
The cost of molecular diagnostic testing as a percentage of revenue increased from 23.3% to 29.5% during the six months ended December 31, 2020 compared to the six months ended December 31, 2019. The increase was primarily driven by the decline in revenue from lower test volumes during the current period, attributable to the continued impact of COVID-19 as lower revenues were generated to cover fixed costs of performing the tests.
50
The cost of pharmaceutical and clinical services as a percentage of revenue decreased from 60.4% to 43.6% during the six months ended December 31, 2020 compared to the six months ended December 31, 2019 primarily due to the sale of the Clinic in February 2020.
Research and Development Expense
| Six Months Ended December 31, | Change | ||||||||||||||||
| (in millions) | 2020 | 2019 (unaudited) | 2020 | ||||||||||||||
| Research and development expense | $ | 35.8 | $ | 40.1 | $ | (4.3) | |||||||||||
| Research and development expense as a % of total revenue | 11.9 | % | 10.5 | % | |||||||||||||
Research and development expense for the six months ended December 31, 2020 decreased compared to the six months ended December 31, 2019 primarily due to decreased personnel expenses due to a decline in headcount and technology related expenses.
Change in the Fair Value of Contingent Consideration
| Six Months Ended December 31, | Change | ||||||||||||||||
| (in millions) | 2020 | 2019 (unaudited) | 2020 | ||||||||||||||
Change in the fair value of contingent consideration | $ | 3.5 | $ | 0.6 | $ | 2.9 | |||||||||||
| Change in the fair value of contingent consideration as a % of total revenue | 1.2 | % | — | % | |||||||||||||
The fair value of contingent consideration for the six months ended December 31, 2020 increased compared to the six months ended December 31, 2019 due to changes in the timing of expected cash payments associated with the contingent consideration related to the Sividon acquisition as a result of revised revenue forecasts.
Selling, General and Administrative Expense
| Six Months Ended December 31, | Change | ||||||||||||||||
| (in millions) | 2020 | 2019 (unaudited) | 2020 | ||||||||||||||
Selling, general, and administrative expense | $ | 256.9 | $ | 269.8 | $ | (12.9) | |||||||||||
| Selling, general, and administrative expense as a % of total revenue | 85.7 | % | 70.7 | % | |||||||||||||
Selling, general and administrative expense decreased for the six months ended December 31, 2020 compared to the six months ended December 31, 2019 primarily due to the Company implementing cost saving measures due to the decline in testing volumes as a result of the impacts of COVID-19 and due to a decline in expense from the sale of the Clinic in February 2020. The cost savings measures implemented by the Company have been partially offset by increased legal and professional service expenses related to our leadership transition and restructuring in the current period. Selling, general and administrative expense as a percentage of total revenue increased for the six months ended December 31, 2020 compared to the six months ended December 31, 2019 primarily due to decreases in revenue outpacing the cost savings measures implemented in the current period.
Other Expense
| Six Months Ended December 31, | Change | ||||||||||||||||
| (in millions) | 2020 | 2019 (unaudited) | 2020 | ||||||||||||||
| Other expense | $ | (6.3) | $ | (4.0) | $ | (2.3) | |||||||||||
Other expense increased for the six months ended December 31, 2020 compared to the six months ended December 31, 2019 due to foreign exchange losses and due to decreased interest income.
51
Income Tax Expense
| Six Months Ended December 31, | Change | ||||||||||||||||
| (in millions) | 2020 | 2019 (unaudited) | 2020 | ||||||||||||||
| Income tax benefit | $ | (41.0) | $ | (4.8) | $ | (36.2) | |||||||||||
| Effective tax rate | 43.6 | % | 14.2 | % | |||||||||||||
Our tax rate is the product of a U.S. federal effective rate of 21% and a blended state income tax rate of approximately 3.5%. Certain significant or unusual items are separately recognized during the period in which they occur and can be a source of variability in the effective tax rates from period to period.
Income tax benefit for the six months ended December 31, 2020 was $41.0 million, and our effective tax rate was 43.6%. The increase in the effective tax rate for the current period compared to the six months ended December 31, 2019 is due primarily to carrying back net operating losses under the provisions of the CARES Act.
Fiscal Years Ended June 30, 2020, 2019 and 2018
Revenue
| Years Ended June 30, | Change | % of Total Revenue | |||||||||||||||||||||||||||||||||||||||||||||
| (In millions) | 2020 | 2019 | 2018 | 2020 | 2019 | 2020 | 2019 | 2018 | |||||||||||||||||||||||||||||||||||||||
| Molecular diagnostic revenue: | |||||||||||||||||||||||||||||||||||||||||||||||
| Hereditary Cancer Testing | $ | 347.4 | $ | 479.7 | $ | 471.4 | $ | (132.3) | $ | 8.3 | 54 | % | 57 | % | 64 | % | |||||||||||||||||||||||||||||||
| GeneSight | 74.1 | 112.6 | 124.9 | (38.5) | (12.3) | 12 | % | 13 | % | 17 | % | ||||||||||||||||||||||||||||||||||||
| Prenatal | 76.7 | 104.9 | — | (28.2) | 104.9 | 12 | % | 12 | % | — | % | ||||||||||||||||||||||||||||||||||||
| Vectra | 39.1 | 48.3 | 55.2 | (9.2) | (6.9) | 6 | % | 6 | % | 7 | % | ||||||||||||||||||||||||||||||||||||
| myChoice CDx | 13.1 | 7.1 | 5.1 | 6.0 | 2.0 | 2 | % | 1 | % | 1 | % | ||||||||||||||||||||||||||||||||||||
| Prolaris | 24.7 | 25.5 | 21.5 | (0.8) | 4.0 | 4 | % | 3 | % | 3 | % | ||||||||||||||||||||||||||||||||||||
| EndoPredict | 10.5 | 10.4 | 8.8 | 0.1 | 1.6 | 2 | % | 1 | % | 1 | % | ||||||||||||||||||||||||||||||||||||
| Other | 1.3 | 0.9 | 3.5 | 0.4 | (2.6) | — | % | — | % | — | % | ||||||||||||||||||||||||||||||||||||
| Total molecular diagnostic revenue | 586.9 | 789.4 | 690.4 | (202.5) | 99.0 | ||||||||||||||||||||||||||||||||||||||||||
| Pharmaceutical and clinical service revenue | 51.7 | 61.7 | 53.3 | (10.0) | 8.4 | 8 | % | 7 | % | 7 | % | ||||||||||||||||||||||||||||||||||||
| Total revenue | $ | 638.6 | $ | 851.1 | $ | 743.7 | $ | (212.5) | $ | 107.4 | 100 | % | 100 | % | 100 | % | |||||||||||||||||||||||||||||||
The Company’s revenues for fiscal year 2020 were significantly impacted by COVID-19 during the third and fourth quarter, as testing volumes declined across the majority of products. The decrease in revenue during fiscal year 2020 was primarily driven by a decrease of $132.3 million in Hereditary cancer testing primarily due an approximate 8% decrease in volumes and an approximate 21% decrease in reimbursement per test, a decrease of $38.5 million in GeneSight revenue due to an approximate 39% decrease in volumes partially offset by an approximate 9% increase in reimbursement per test, a decrease of $28.2 million in Prenatal revenue due to an approximate 30% decrease in reimbursement per test, a $9.2 million decrease in Vectra revenue due to an approximate 18% decrease in volumes, and a $10.0 million decrease in Pharmaceutical and clinical service revenue primarily as a result of selling the Clinic in February 2020.
In 2019, the increase in revenue was primarily due to the inclusion of $104.9 million in Prenatal revenue due to the acquisition of Counsyl, an increase of $8.4 million in Pharmaceutical and clinical service revenue due to increased volumes, an increase of $8.3 million in Hereditary cancer testing due to increased volumes, a $4.0 million increase in Prolaris revenue due to increased volumes and reimbursement, and a $1.6 million increase in EndoPredict revenue due to increased volumes. The increases were partially offset by decreases of $12.3 million in GeneSight revenue due to reduced reimbursement, and a $6.9 million decrease in Vectra revenue due to lower volumes.
52
Cost of Sales
| Years Ended June 30, | Change | |||||||||||||||||||||||||||||||
| (in millions) | 2020 | 2019 | 2018 | 2020 | 2019 | |||||||||||||||||||||||||||
| Cost of molecular diagnostic testing | $ | 157.5 | $ | 168.2 | $ | 148.7 | $ | (10.7) | $ | 19.5 | ||||||||||||||||||||||
| Cost of molecular diagnostic testing as a % of revenue | 26.8 | % | 21.3 | % | 21.5 | % | ||||||||||||||||||||||||||
| Cost of pharmaceutical and clinical services | $ | 28.6 | $ | 32.8 | $ | 28.5 | $ | (4.2) | $ | 4.3 | ||||||||||||||||||||||
| Cost of pharmaceutical and clinical services as a % of revenue | 55.3 | % | 53.2 | % | 53.5 | % | ||||||||||||||||||||||||||
Cost of molecular diagnostic testing as a percentage of revenue increased from 21.3% to 26.8% during fiscal year 2020 compared to fiscal year 2019. The increase was primarily driven by the decline in revenue from lower test volumes during the period, attributable to the impact of COVID-19 primarily during the fourth quarter as lower revenues were generated to cover fixed costs, and due to reduction of reimbursement related to Hereditary cancer testing and Prenatal. Cost of pharmaceutical and clinical services decreased $4.2 million during fiscal year 2020 compared to fiscal year 2019 primarily due to the sale of the Clinic in February 2020
Cost of molecular diagnostic testing as a percentage of revenue decreased slightly from 21.5% to 21.3% during fiscal year 2019 compared to fiscal year 2018. The decrease was primarily driven by the implementation of efficiency programs in our DNA, RNA, and protein-based laboratories. These decreases were partially offset by lower gross margins associated with the Counsyl business and reduction of reimbursement related to Hereditary cancer testing and GeneSight.
Research and Development Expense
| Years Ended June 30, | Change | ||||||||||||||||||||||||||||
| (in millions) | 2020 | 2019 | 2018 | 2020 | 2019 | ||||||||||||||||||||||||
| Research and development expense | $ | 77.2 | $ | 85.9 | $ | 70.8 | $ | (8.7) | $ | 15.1 | |||||||||||||||||||
| Research and development expense as a % of total revenue | 12.1 | % | 10.1 | % | 9.5 | % | |||||||||||||||||||||||
In fiscal year 2020, research and development expense decreased compared to the fiscal year 2019 primarily due to synergies recognized as part of the integration of the Counsyl business partially offset by an additional month of Counsyl business expenses included in the current year.
In fiscal year 2019, research and development expense increased compared to fiscal year 2018 primarily driven by $17.3 million in costs related to the inclusion of Counsyl. This increase was partially offset by a reduction in costs related to internal development of existing products.
Change in the Fair Value of Contingent Consideration
| Years Ended June 30, | Change | ||||||||||||||||||||||||||||
| (in millions) | 2020 | 2019 | 2018 | 2020 | 2019 | ||||||||||||||||||||||||
| Change in the fair value of contingent consideration | $ | (2.8) | $ | 1.1 | $ | (61.2) | $ | (3.9) | $ | 62.3 | |||||||||||||||||||
| Change in the fair value of contingent consideration as a % of total revenue | (0.4) | % | 0.1 | % | (8.2) | % | |||||||||||||||||||||||
In fiscal year 2020, the decrease in the change in fair value of contingent consideration compared to fiscal year 2019 is due to changes in timing of expected cash payments associated with the contingent consideration related to the Sividon acquisition as a result of revised revenue forecasts.
In fiscal year 2019, the increase in the change in fair value of contingent consideration compared to the prior year is primarily due to an increase in the fair value of contingent consideration related to the Sividon acquisition as well as the one-time benefit received in the prior year resulting from not having to pay the clinical trial milestone associated with the GUIDED study.
53
Selling, General and Administrative Expense
| Years Ended June 30, | Change | ||||||||||||||||||||||||||||
| (in millions) | 2020 | 2019 | 2018 | 2020 | 2019 | ||||||||||||||||||||||||
| Selling, general, and administrative expense | $ | 510.1 | $ | 555.5 | $ | 435.0 | $ | (45.4) | $ | 120.5 | |||||||||||||||||||
| Selling, general, and administrative expense as a % of total revenue | 79.9 | % | 65.3 | % | 58.5 | % | |||||||||||||||||||||||
In fiscal year 2020, the decrease in selling, general, and administrative expense compared to the prior year is primarily due to the Company implementing cost saving measures during the fourth quarter due to the decline in testing volumes as a result of the impacts of COVID-19 and a reduction in costs related to synergies recognized relating to the integration of the Counsyl business.
In fiscal year 2019, the increase in selling, general, and administrative expense compared to the prior year is primarily due to $55.0 million in costs related to the inclusion of Counsyl, $22.1 million of Counsyl amortization of intangible assets, $20.8 million in costs related to the acquisition and integration of Counsyl, $9.1 million related to the settlement of the complaint filed by a qui tam relator, and additional spend related to improving our IT infrastructure.
Goodwill and Intangible Asset Impairment Charges
| Years Ended June 30, | Change | ||||||||||||||||||||||||||||
| (in millions) | 2020 | 2019 | 2018 | 2020 | 2019 | ||||||||||||||||||||||||
| Goodwill and intangible asset impairment charges | $ | 99.7 | $ | — | $ | — | $ | 99.7 | $ | — | |||||||||||||||||||
In fiscal year 2020, goodwill and intangible asset impairment charges increased compared to the same period in the prior year due to the Company recognizing goodwill impairment charges related to the Myriad Autoimmune and Clinic reporting units and charges related to the abandonment of an in-process research and development intangible asset in the current year. There were no impairments recognized in the prior fiscal years.
Other Income (Expense)
| Years Ended June 30, | Change | ||||||||||||||||||||||||||||
| (in millions) | 2020 | 2019 | 2018 | 2020 | 2019 | ||||||||||||||||||||||||
| Other income (expense) | $ | 8.4 | $ | (7.6) | $ | (1.8) | $ | 16.0 | $ | (5.8) | |||||||||||||||||||
In fiscal year 2020, the increase in other income (expense) compared to fiscal year 2019 is primarily driven by the receipt of stimulus funds from the CARES Act in the amount of $14.6 million, the gain recognized on the sale of the Clinic, income from a state grant, and a decrease in interest expense.
In fiscal year 2019, the increase in other expense compared to the prior year was primarily driven by an increase in interest expense related to the debt incurred to fund the acquisition of Counsyl. This was partially offset by increased interest income.
Income Tax Expense
| Years Ended June 30, | Change | ||||||||||||||||||||||||||||
| (in millions) | 2020 | 2019 | 2018 | 2020 | 2019 | ||||||||||||||||||||||||
| Income tax benefit | $ | (23.7) | $ | (4.4) | $ | (13.0) | $ | (19.3) | $ | 8.6 | |||||||||||||||||||
| Effective tax rate | 10.6 | % | (14107.7) | % | (10.7) | % | |||||||||||||||||||||||
Our tax rate is the product of a U.S. federal effective rate of 21% and a blended state income tax rate of approximately 3.5%. Certain significant or unusual items are separately recognized during the period in which they occur and can be a source of variability in the effective tax rates from period to period.
Income tax benefit for the fiscal year ended June 30, 2020 is $23.7 million for an effective tax benefit rate of 10.6%. The change in the effective rate as compared to the prior year is due to the prior year being near break-even, resulting in a very large effective rate. Differences related to the recognition of the tax effect of equity compensation expense from the disqualification of incentive stock options, asset impairment, uncertain tax benefits and changes in valuation allowance also impacted the current and prior year effective tax rates.
54
Income tax benefit for the fiscal year ended June 30, 2019 is $4.4 million for an effective tax rate of (14,107.7%). The decrease in the effective rate as compared to the prior year is due to a $32.0 million one-time Tax Act benefit in the prior year, disregarded election of foreign entities, amended filing and method changes, and statute lapse of uncertain tax positions. Differences related to the recognition of the tax effect of equity compensation expense from the disqualification of incentive stock options also impacted the current and prior year effective tax rate.
Liquidity and Capital Resources
Our primary sources of liquidity are our cash, cash equivalents and marketable investment securities, our cash flows from operations and amounts available under our Amended Facility. Additionally, we recently announced that we are exploring strategic alternatives for our Myriad RBM, Myriad Dermatology, and Myriad Autoimmune business units. If we choose to sell these or other assets, the proceeds will also provide additional liquidity. Our capital deployment strategy focuses on use of resources in the key areas of research and development, infrastructure, debt repayment, acquisitions, and the repurchase of our common stock. We believe that investing organically through research and development or acquisitively to support business strategy provides the best return on invested capital.
We believe that our existing capital resources and the cash to be generated from future sales, taking into consideration the potential further impacts of COVID-19 on our operations, will be sufficient to meet our projected operating requirements and repay the outstanding Amended Facility, which matures on July 31, 2023 and which has no scheduled principal payments prior to that date. Our available capital resources, however, may be consumed more rapidly than currently expected, and we may need or want to raise additional financing. We may not be able to secure such financing in a timely manner or on favorable terms, if at all. We are subject to financial covenants as part of our outstanding Amended Facility. It is possible that we could be in violation of certain financial covenants contained in the Amended Facility in the future. We may seek waivers or amendments from our lenders in order to avoid a future potential covenant violation, in addition to taking other potential actions. For example, on February 22, 2021, we amended our credit facility to waive compliance with the leverage ratio covenant and the interest coverage ratio covenant through the quarter ended March 31, 2022 and to also lower the minimum liquidity covenant through the same period. If we were unable to comply with the covenants in the future, that could result in an increase in the rate of interest and limits on our ability to incur certain additional indebtedness and could potentially cause the loan repayment to be accelerated. Without additional funds, we may be forced to delay, scale back or eliminate some of our sales and marketing efforts, research and development activities, or other operations and potentially delay development of our diagnostic tests in an effort to provide sufficient funds to continue our operations. If any of these events occurs, our ability to achieve our development and commercialization goals could be adversely affected.
The Amended Facility restricts the Company from borrowing under the revolving credit facility if unrestricted cash and cash equivalents exceed $150.0 million, unless such borrowings are in connection with acquisitions. The Amended Facility also includes an immediate reduction in the commitment to $300.0 million and further reduction to $250.0 million by September 30, 2021, and includes terms for further reductions, or mandatory prepayments of revolving loans, in the event of certain asset sales, which could limit our borrowing capacity, or reduce liquidity, in future periods. The Amended Facility allows the Company to keep the net cash proceeds of material asset sales received above certain dollar thresholds without corresponding mandatory prepayments or commitment reductions.
Additionally, the COVID-19 pandemic and resulting global disruptions have caused significant volatility in financial markets. This disruption may affect asset valuations resulting in impairment charges, may contribute to potential defaults in our accounts receivable, and affect the availability of lease and financing credit as well as other segments of the credit markets. However, due to the continuing evolving global situation, it is not possible to predict whether unanticipated consequences of the pandemic are reasonably likely to materially affect our liquidity and capital resources in the future.
As of December 31, 2020, the Company had approximately $21.4 million of non-cancelable contractual purchase obligations with varying terms over the next four years, with most purchase obligations due within the next 12 months.
55
The following table represents the balances of cash, cash equivalents and marketable investment securities:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| Cash and cash equivalents | $ | 117.0 | $ | 163.7 | $ | 93.2 | $ | 110.9 | |||||||||||||||
| Marketable investment securities | 33.7 | 54.1 | 43.7 | 69.7 | |||||||||||||||||||
| Long-term marketable investment securities | 21.0 | 37.0 | 54.9 | 30.7 | |||||||||||||||||||
Cash, cash equivalents and marketable investment securities | $ | 171.7 | $ | 254.8 | $ | 191.8 | $ | 211.3 | |||||||||||||||
In the transition period ended December 31, 2020, the decrease in cash, cash equivalents and marketable investment securities was primarily driven by the Company using $73.7 million in cash for operating activities.
In fiscal year 2020, the increase in cash, cash equivalents and marketable investment securities was primarily driven by $60.7 million in cash provided by operating activities and $21.3 million from the sale of a subsidiary. These increases were partially offset by $8.6 million in payments towards our Amended Facility.
In fiscal year 2019, the decrease in cash, cash equivalents and marketable investment securities was driven by $286.4 million in cash used in investing activities primarily related to $278.5 million of cash used in the acquisition of Counsyl. This decrease was partially offset by an increase in cash of $182.3 million related to financing activities primarily related to a $225.0 million net increase in proceeds from the Amended Facility and an increase in cash provided by operating activities of $83.7 million.
The following table represents the condensed cash flow statement:
| Transition Period Ended December 31, | Six Months Ended December 31, (unaudited) | Years Ended June 30, | |||||||||||||||||||||||||||
| (in millions) | 2020 | 2019 | 2020 | 2019 | 2018 | ||||||||||||||||||||||||
| Cash flows from operating activities | $ | (73.7) | $ | 13.9 | $ | 60.7 | $ | 83.7 | $ | 115.9 | |||||||||||||||||||
| Cash flows from investing activities | 28.0 | (14.3) | 19.3 | (286.4) | (11.6) | ||||||||||||||||||||||||
| Cash flows from financing activities | (2.1) | (11.2) | (10.0) | 182.3 | (95.0) | ||||||||||||||||||||||||
Effect of foreign exchange rates on cash and cash equivalents | 1.1 | 1.1 | 0.5 | 2.7 | (0.8) | ||||||||||||||||||||||||
| Change in cash and cash equivalents classified as held for sale | — | (1.5) | — | — | — | ||||||||||||||||||||||||
| Net increase (decrease) in cash and cash equivalents | (46.7) | (12.0) | 70.5 | (17.7) | 8.5 | ||||||||||||||||||||||||
| Cash and cash equivalents at the beginning of the period | 163.7 | 93.2 | 93.2 | 110.9 | 102.4 | ||||||||||||||||||||||||
| Cash and cash equivalents at the end of the period | $ | 117.0 | $ | 81.2 | $ | 163.7 | $ | 93.2 | $ | 110.9 | |||||||||||||||||||
Cash Flows from Operating Activities
In the transition period ended December 31, 2020, the primary driver of the decrease in cash flows from operating activities was a decline in revenues of $81.6 million primarily due to factors related to the COVID-19 impact on volumes and a decline in the average expected reimbursement per test. Our Hereditary Cancer products were impacted the most from these factors with a decrease of $62.9 million in revenues for the six months ended December 31, 2020 compared to the six months ended December 31, 2019. Revenue from Hereditary Cancer products declined primarily due to an approximate 17% decrease in volumes and an approximate 14% decrease in average reimbursement per test. Decreases in cash flows from operations was also due to an increase in trade receivables in the transition period due to delays in receipt of reimbursements compared to the prior period. The decrease in cash flows from operations was partially offset by cost reductions due to lower volumes and cost saving initiatives.
In fiscal year 2020, the primary driver of the decrease in cash flows from operating activities was the $104.4 million decrease in net income (loss), excluding the impact of the impairment of goodwill and intangible assets, and a decrease in the non-cash adjustment related to deferred income taxes of $74.4 million compared to 2019. These changes were partially offset by a $152.2 million net change in assets and liabilities.
56
In fiscal year 2019, the primary driver of the decrease in cash flows from operating activities was the $107.5 million decrease in net income excluding contingent consideration and a $45.6 million change in assets and liabilities. These were partially offset by a $142.1 million increase related to non-cash charges.
Cash Flows from Investing Activities
In the transition period ended December 31, 2020, the increase in cash flows from investing activities was primarily driven by the use of proceeds from the maturities of marketable investment securities to fund operations compared to reinvesting such proceeds in purchases of marketable securities in prior years.
In fiscal year 2020, the increase in cash flows from investing activities was primarily driven by the $278.5 million of cash used for the purchase of Counsyl in the prior fiscal year as well as $21.3 million in proceeds from the sale of a subsidiary in the current fiscal year.
In fiscal year 2019, the decrease in cash flows from investing activities was primarily driven by the $278.5 million of cash used for the purchase of Counsyl.
Cash Flows from Financing Activities
In the transition period ended December 31, 2020, the decrease in cash flows from financing activities was driven primarily by repayments of the Amended Facility completed in the prior period compared to no repayments in the current period.
In fiscal year 2020, the decrease in cash flows from financing activities was driven primarily by the prior year’s net proceeds from the Amended Facility of $225.0 million, offset by the prior year’s $50.0 million reduction in cash used for share repurchases, compared to the current year repayment of the Amended Facility of $8.6 million.
In fiscal year 2019, the increase in cash flows from financing activities was driven primarily by a $225.0 million increase in net proceeds from the Amended Facility and the prior year’s $42.4 million payment of contingent consideration related to the Assurex acquisition. These were partially offset by a $50.0 million reduction in cash used for share repurchase and $28.2 million decrease in proceeds from common stock issued under share-based compensation plans.
Effects of Inflation
We do not believe that inflation has had a material impact on our business, revenues, or operating results during the periods presented.
Market, Industry and Other Data
This Transition Report on Form 10-K contains estimates, projections and other information concerning our industry, our business and relevant molecular diagnostics markets, including data regarding the estimated size of relevant molecular diagnostic markets, patient populations, and the perceptions and preferences of patients and physicians regarding certain therapies, as well as data regarding market research and estimates. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources that we believe to be reliable. In some cases, we do not expressly refer to the sources from which this data is derived. In that regard, when we refer to one or more sources of this type of data in any paragraph, you should assume that other data of this type appearing in the same paragraph is derived from the same sources, unless otherwise expressly stated or the context otherwise requires.
57
Critical Accounting Policies
Critical accounting policies are those policies which are both important to the portrayal of a company’s financial condition and results and require management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. Our critical accounting policies are as follows:
•revenue recognition;
•goodwill; and
•income taxes.
Revenue Recognition. Revenue is recognized when, or as, performance obligations under the terms of a contract are satisfied, which occurs when control of the promised products or services is transferred to a customer. We exclude sales, use, value-added, and other taxes we collect on behalf of third parties from revenue. Revenue is measured as the amount of consideration we expect to receive in exchange for transferring products or services to a customer.
We generate revenue by performing molecular diagnostic testing and pharmaceutical and clinical services and perform our obligation under a contract with a customer by processing those diagnostic tests and communicating the test results to customers, in exchange for consideration from the customer. Revenue from the sale of molecular diagnostic tests and pharmaceutical and clinical services is recorded at the estimated transaction price. We have determined that the communication of test results or the completion of clinical and pharmaceutical services indicates transfer of control for revenue recognition purposes. We have the right to bill our customers upon the completion of performance obligations and thus does not record contract assets. Occasionally customers make payments prior to our performance of our contractual obligations. When this occurs, we record a contract liability as deferred revenue.
Significant judgments are required in determining the transaction price and satisfying performance obligations under the revenue standard. In determining the transaction price, we estimate the expected amount of consideration as revenue. We apply this method consistently for similar contracts when estimating the effect of any uncertainty on an amount of variable consideration to which it will be entitled. An estimate of transaction price does not include any estimated amount of variable consideration that is constrained. We consider all the information (historical, current, and forecast) that is reasonably available to identify possible consideration amounts. To determine our estimated transaction price, we apply the expected value method for sales where we have a large number of contracts with similar characteristics. We then consider the probability of the variable consideration for each possible scenario. We have significant experience with historical discount patterns and use this experience to estimate transaction prices.
The estimate of revenue is affected by assumptions in payor mix and in payor behavior such as changes in payor collections, current customer contractual requirements, and experience with ultimate collection from third-party payors. When assessing the total consideration for insurance carriers and patients, revenues are further constrained for estimated refunds. The Company reserves certain amounts in accrued liabilities in the consolidated balance sheets in anticipation of request for refunds of payments made previously by insurance carriers, which are accounted for as reductions in revenues in the Consolidated Statements of Operations and Comprehensive Income (Loss).
Goodwill. We test goodwill for impairment on an annual basis and in the interim by reporting unit if events and circumstances indicate that goodwill may be impaired. The events and circumstances that are considered include business climate and market conditions, legal factors, operating performance indicators and competition. Impairment of goodwill is evaluated on a qualitative basis before calculating the fair value of the reporting unit. If the qualitative assessment suggests that impairment is more likely than not, a quantitative impairment analysis is performed. The quantitative analysis involves comparison of the fair value of a reporting unit with its carrying amount. The valuation of a reporting unit requires judgment in estimating future cash flows, discount rates, residual growth rates and other factors. In making these judgments, we evaluate the financial health of our business, including such factors as industry performance, market saturation and opportunity, changes in technology and operating cash flows. Changes in our forecasts or decreases in the value of our common stock could cause book value of reporting units to exceed their fair values. If the carrying amount of a reporting unit exceeds its fair value, an impairment loss is recognized in an amount equal to that excess, limited to the total amount of goodwill allocated to that reporting unit. If an event occurs that would cause a revision to the estimates and assumptions used in analyzing the value of the goodwill, the revision could result in a non-cash impairment charge that could have a material impact on the financial results.
58
As of December 31, 2020, we have recorded goodwill of $329.2 million on our Consolidated Balance Sheet. Of this goodwill, $272.3 million is related to our molecular diagnostic segment for Myriad Autoimmune, Myriad Mental Health (formerly known as Myriad Neuroscience), Myriad International, and Myriad Women's Health reporting units and $56.9 million for the Myriad RBM reporting unit related to our other segment (see Note 15 for segment descriptions). We qualitatively evaluated the Myriad RBM reporting unit for impairment noting no indicators of impairment. For the remaining four reporting units, quantitative impairment analyses were completed to evaluate for impairment. We measured the fair value of Myriad Autoimmune, Myriad Mental Health (formerly known as Myriad Neuroscience), Myriad International, and Myriad Women's Health reporting units utilizing the market approach and also utilizing the discounted cash flow method under the income approach. The income approach considered management's business plans and projections as the basis for expected cash flow for the next thirteen to fifteen years and a 2% long-term growth rate, and profitability as the significant estimate used in the analysis.
The weighted average discount rate and fair value of each reporting unit in excess of its carrying valuing using the assumptions above are as follows:
| Weighted Average Discount Rate | % Fair Value in Excess of Carrying Value | ||||||||||
| Myriad Mental Health | 11.5 | % | 12 | % | |||||||
| Myriad Autoimmune | 13.5 | % | 34 | % | |||||||
| Myriad International | 12.0 | % | 166 | % | |||||||
| Myriad Women's Health | 11.5 | % | 47 | % | |||||||
Income Taxes. Our income tax provision is based on income before taxes and is computed using the liability method in accordance with Accounting Standards Codification (“ASC”) 740 – Income Taxes. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using tax rates projected to be in effect for the year in which the differences are expected to reverse. Significant estimates are required in determining our provision for income taxes. Some of these estimates are based on interpretations of existing tax laws or regulations, or the expected results from any future tax examinations. Various internal and external factors may have favorable or unfavorable effects on our future provision for income taxes. Those factors include, but are not limited to, changes in tax laws, regulations and/or rates, the results of any future tax examinations, changing interpretations of existing tax laws or regulations, changes in estimates of prior years’ items, past levels of research and development spending, acquisitions, changes in our corporate structure, and changes in overall levels of income before taxes all of which may result in periodic revisions to our provision for income taxes.
Developing our provision for income taxes, including our effective tax rate and analysis of potential uncertain tax positions, if any, requires significant judgment and expertise in federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and any estimated valuation allowance we deem necessary to offset deferred tax assets. If we do not maintain taxable income from operations in future periods, we may increase the valuation allowance for our deferred tax assets and record material adjustments to our income tax expense. Our judgment and tax strategies are subject to audit by various taxing authorities. While we believe we have provided adequately for our uncertain income tax positions in our consolidated financial statements, adverse determination by these taxing authorities could have a material adverse effect on our consolidated financial condition, results of operations or cash flows. Interest and penalties on income tax items are included as a component of overall income tax expense.
Recent Accounting Pronouncements
See Note 1 to the Consolidated Financial Statements included in Item 8 of this Report for a description of recent accounting pronouncements.
59
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We maintain an investment portfolio in accordance with our written investment policy. The primary objectives of our investment policy are to preserve principal, maintain proper liquidity to meet operating needs and maximize yields. Our investment policy specifies credit quality standards for our investments and limits the amount of credit exposure to any single issue, issuer or type of investment.
Our investments consist of debt securities of various types and maturities of five years or less, with an average maturity of two years. These securities are classified as available-for-sale. Available-for-sale securities are recorded on the balance sheet at fair market value with unrealized gains or losses reported as part of Accumulated Other Comprehensive Income (Loss). Realized gains and losses on investment security transactions are reported on the specific-identification method. Dividend and interest income are recognized when earned. A decline in the market value of any available-for-sale security below cost that is deemed other-than-temporary results in a charge to earnings and establishes a new cost basis for the security.
Although our investment policy guidelines are intended to ensure the preservation of principal, market conditions can result in high levels of uncertainty. Our ability to trade or redeem the marketable investment securities in which we invest, including certain corporate bonds, may become difficult. Valuation and pricing of these securities can also become variable and subject to uncertainty.
As of December 31, 2020, we had $0.8 million in unrealized gains in our investment portfolio. For the transition period ended December 31, 2020 we have experienced fluctuations in our portfolio value primarily from our investments in corporate bonds. If interest rates rise, the market value of our investments may decline, which could result in a realized loss if we are forced to sell an investment before its scheduled maturity. A hypothetical increase in interest rates by 25 basis points would have resulted in an increase in the fair value of our net investment position of approximately $0.3 million as of December 31, 2020 and 2019, respectively. We do not utilize derivative financial instruments to manage our interest rate risks.
We also maintain a long-term debt balance that has exposure to market risk for changes in interest rates. Our long-term debt balance is carried at amortized cost and fluctuations in interest rates do not impact our consolidated financial statements. However, the fair value of our debt will generally fluctuate with movements of interest rates, including in periods of declining rates of interest. If interest rates rise, we would incur additional interest expense related to the long-term debt balance.
We may be exposed to fluctuations in foreign currencies with regard to certain agreements with service providers. Depending on the strengthening or weakening of the United States dollar, realized and unrealized currency fluctuations could be significant.
60
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
MYRIAD GENETICS, INC.
| Page | |||||
| 62 | |||||
| 64 | |||||
| 65 | |||||
| 66 | |||||
| 67 | |||||
| 68 | |||||
| 69 | |||||
61
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Myriad Genetics, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Myriad Genetics, Inc. and subsidiaries (the Company) as of December 31, 2020, June 30, 2020 and June 30, 2019, the related consolidated statements of operations, comprehensive income (loss), stockholders' equity and cash flows for the six month period ended December 31, 2020 and each of the three years in the period ended June 30, 2020, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2020, June 30, 2020 and 2019, and the results of its operations and its cash flows for the six month period ended December 31, 2020 and each of the three years in the period ended June 30, 2020, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the Company's internal control over financial reporting as of December 31, 2020, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated March 16, 2021 expressed an adverse opinion thereon.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matters below, providing separate opinions on the critical audit matters or on the accounts or disclosures to which they relate.
| Impairment evaluation of goodwill | ||||||||
| Description of the Matter | At December 31, 2020, the Company’s goodwill balance was $329.2 million. As discussed in Note 5 of the financial statements, goodwill is tested for impairment at least annually or more frequently if indicators of impairment require the performance of an interim impairment assessment. Auditing management’s impairment tests was complex and highly judgmental due to the significant estimation required in determining the fair value of the reporting units containing goodwill. Specifically, the fair value estimates of the reporting units were sensitive to significant assumptions including the estimation of expected cash flows, discount rates, and residual growth rates. The fair value estimates of the reporting units are affected by such factors as industry performance, market saturation and opportunity, changes in technology and operating cash flows. | |||||||
62
| How We Addressed the Matter in Our Audit | We obtained an understanding, evaluated the design and tested the operating effectiveness of controls over the Company’s goodwill impairment review processes. For example, we tested controls over the quantitative impairment analyses of goodwill, including the valuation models and underlying assumptions used to develop such estimates. To test the estimated fair value of the Company’s reporting units, we performed audit procedures that included, among others, evaluating the Company’s valuation methodology used, evaluating the prospective financial information utilized in the valuations, and involving our valuation specialists to assist in testing certain significant assumptions described above, such as residual growth rates and discount rates including company risk premiums. We assessed the historical accuracy of management’s estimates and performed sensitivity analyses of significant assumptions to evaluate the changes in the fair value of the reporting units that would result from changes in the assumptions. | |||||||
| Measurement of molecular diagnostic testing revenue | ||||||||
| Description of the Matter | During the six month transition period ended December 31, 2020, the Company’s molecular diagnostic testing revenue was $279.6 million. As discussed in Note 1 of the consolidated financial statements, molecular diagnostic testing revenue is recognized when the performance obligation is complete. Auditing the measurement of the Company’s molecular diagnostic testing revenue was complex and judgmental due to the significant estimation required in estimating the amount that will be collected for each test. In particular, the estimate of revenue is affected by assumptions related to payors such as changes in payor mix, payor collections, current customer contractual requirements, and experience with ultimate collection from third-party payors. | |||||||
| How We Addressed the Matter in Our Audit | We obtained an understanding and evaluated the design and tested the operating effectiveness of controls over the Company’s revenue recognition process. As part of our testing, we considered controls over management’s review of the significant assumptions above and inputs used in calculating the estimated amount that would be collected for each test and tested management’s controls to compare actual payments received to previously forecasted activity. We also tested controls used by management to compare the current and historical data used in making the estimates for completeness and accuracy. Our audit procedures over the Company’s molecular diagnostic testing revenue included, among others, assessing valuation methodologies and models and testing the significant assumptions above and the underlying data used by the Company in its analysis. We agreed transactions selected for testing back to the actual customer contract terms. We compared the significant assumptions above and inputs used by management to changes in the Company’s contracted rates, third-party payor collection trends, and other relevant factors. We assessed the historical accuracy of the cash collections used in the Company’s revenue models and assessed the completeness of adjustments to estimates of future cash collections as a result of significant contract amendments, changes in collection trends and changes in payor behavior. | |||||||
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2006.
Salt Lake City, UT
March 16, 2021
63
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Balance Sheets
(in millions)
| December 31, | June 30, | ||||||||||||||||
| 2020 | 2020 | 2019 | |||||||||||||||
| ASSETS | |||||||||||||||||
| Current assets: | |||||||||||||||||
| Cash and cash equivalents | $ | $ | $ | ||||||||||||||
| Marketable investment securities | |||||||||||||||||
| Prepaid expenses | |||||||||||||||||
| Inventory | |||||||||||||||||
| Trade accounts receivable | |||||||||||||||||
| Prepaid taxes | |||||||||||||||||
| Other receivables | |||||||||||||||||
| Total current assets | |||||||||||||||||
| Property, plant and equipment, net | |||||||||||||||||
| Operating lease right-of-use assets | |||||||||||||||||
| Long-term marketable investment securities | |||||||||||||||||
| Intangibles, net | |||||||||||||||||
| Goodwill | |||||||||||||||||
| Other assets | |||||||||||||||||
| Total assets | $ | $ | $ | ||||||||||||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | |||||||||||||||||
| Current liabilities: | |||||||||||||||||
| Accounts payable | $ | $ | $ | ||||||||||||||
| Accrued liabilities | |||||||||||||||||
| Current maturities of operating lease liabilities | |||||||||||||||||
| Deferred revenue | |||||||||||||||||
| Total current liabilities | |||||||||||||||||
| Unrecognized tax benefits | |||||||||||||||||
| Long-term deferred taxes | |||||||||||||||||
| Long-term debt | |||||||||||||||||
| Noncurrent operating lease liabilities | |||||||||||||||||
| Other long-term liabilities | |||||||||||||||||
| Total liabilities | |||||||||||||||||
| Commitments and contingencies | |||||||||||||||||
| Stockholders’ equity: | |||||||||||||||||
Common stock, | |||||||||||||||||
| Additional paid-in capital | |||||||||||||||||
| Accumulated other comprehensive income (loss) | ( | ( | ( | ||||||||||||||
| Retained earnings (accumulated deficit) | ( | ( | |||||||||||||||
| Total Myriad Genetics, Inc. stockholders' equity | |||||||||||||||||
| Non-controlling interest | |||||||||||||||||
| Total stockholders' equity | |||||||||||||||||
| Total liabilities and stockholders’ equity | $ | $ | $ | ||||||||||||||
64
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Statements of Operations
(in millions, except per share amounts)
| Six-month Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| 2020 | 2020 | 2019 | 2018 | ||||||||||||||||||||
| Molecular diagnostic testing | $ | $ | $ | $ | |||||||||||||||||||
| Pharmaceutical and clinical services | |||||||||||||||||||||||
| Total revenue | |||||||||||||||||||||||
| Costs and expenses: | |||||||||||||||||||||||
| Cost of molecular diagnostic testing | |||||||||||||||||||||||
| Cost of pharmaceutical and clinical services | |||||||||||||||||||||||
| Research and development expense | |||||||||||||||||||||||
| Change in the fair value of contingent consideration | ( | ( | |||||||||||||||||||||
| Selling, general, and administrative expense | |||||||||||||||||||||||
| Goodwill and intangible asset impairment charges | |||||||||||||||||||||||
| Total costs and expenses | |||||||||||||||||||||||
| Operating income (loss) | ( | ( | |||||||||||||||||||||
| Other income (expense): | |||||||||||||||||||||||
| Interest income | |||||||||||||||||||||||
| Interest expense | ( | ( | ( | ( | |||||||||||||||||||
| Other | ( | ( | |||||||||||||||||||||
| Total other income (expense) | ( | ( | ( | ||||||||||||||||||||
| Income (loss) before income tax | ( | ( | |||||||||||||||||||||
| Income tax benefit | ( | ( | ( | ( | |||||||||||||||||||
| Net income (loss) | ( | ( | |||||||||||||||||||||
| Net loss attributable to non-controlling interest | ( | ( | ( | ||||||||||||||||||||
| Net income (loss) attributable to Myriad Genetics, Inc. stockholders | $ | ( | $ | ( | $ | $ | |||||||||||||||||
| Earnings (loss) per share: | |||||||||||||||||||||||
| Basic | $ | ( | $ | ( | $ | $ | |||||||||||||||||
| Diluted | $ | ( | $ | ( | $ | $ | |||||||||||||||||
| Weighted average shares outstanding: | |||||||||||||||||||||||
| Basic | |||||||||||||||||||||||
| Diluted | |||||||||||||||||||||||
See accompanying notes to consolidated financial statements.
65
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Statements of Comprehensive Income (Loss)
(in millions)
| Six-month Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| 2020 | 2020 | 2019 | 2018 | ||||||||||||||||||||
| Net income (loss) attributable to Myriad Genetics, Inc. stockholders | $ | ( | $ | ( | $ | $ | |||||||||||||||||
| Unrealized gain (loss) on available-for-sale securities, net of tax | ( | ( | |||||||||||||||||||||
| Change in pension liability | |||||||||||||||||||||||
| Change in foreign currency translation adjustment | ( | ( | |||||||||||||||||||||
| Comprehensive income (loss) | $ | ( | $ | ( | $ | $ | |||||||||||||||||
See accompanying notes to consolidated financial statements.
66
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Statements of Stockholders’ Equity
(in millions)
| Common stock | Additional paid-in capital | Accumulated other comprehensive income (loss) | Retained earnings (accumulated deficit) | Myriad Genetics, Inc. Stockholders’ equity | |||||||||||||||||||||||||
| BALANCES AT JUNE 30, 2017 | $ | $ | $ | ( | $ | ( | $ | ||||||||||||||||||||||
| Issuance of common stock under share-based compensation plans, net of shares exchanged for withholding tax | — | — | — | ||||||||||||||||||||||||||
| Share-based payment expense | — | — | — | ||||||||||||||||||||||||||
| Net income | — | — | — | ||||||||||||||||||||||||||
| Other comprehensive income, net of tax | — | — | — | ||||||||||||||||||||||||||
| BALANCES AT JUNE 30, 2018 | ( | ||||||||||||||||||||||||||||
| Issuance of common stock under share-based compensation plans, net of shares exchanged for withholding tax | — | — | — | ||||||||||||||||||||||||||
| Share-based payment expense | — | — | — | ||||||||||||||||||||||||||
| Repurchase and retirement of common stock | — | ( | — | ( | ( | ||||||||||||||||||||||||
| Net income | — | — | — | ||||||||||||||||||||||||||
| Other comprehensive loss, net of tax | — | — | ( | — | ( | ||||||||||||||||||||||||
| BALANCES AT JUNE 30, 2019 | ( | ||||||||||||||||||||||||||||
| Issuance of common stock under share-based compensation plans, net shares exchanged for withholding tax | — | — | — | ||||||||||||||||||||||||||
| Share-based payment expense | — | — | — | ||||||||||||||||||||||||||
| Net loss | — | — | — | ( | ( | ||||||||||||||||||||||||
| Reclassification out of accumulated other comprehensive loss upon the deconsolidation of a subsidiary | — | — | — | ||||||||||||||||||||||||||
| Other comprehensive income, net of tax | — | — | — | ||||||||||||||||||||||||||
| BALANCES AT JUNE 30, 2020 | ( | ( | |||||||||||||||||||||||||||
| Issuance of common stock under share-based compensation plans, net shares exchanged for withholding tax | ( | — | — | ( | |||||||||||||||||||||||||
| Share-based payment expense | — | — | — | ||||||||||||||||||||||||||
| Net loss | — | — | — | ( | ( | ||||||||||||||||||||||||
| Other comprehensive income, net of tax | — | — | — | ||||||||||||||||||||||||||
| BALANCES AT DECEMBER 31, 2020 | $ | $ | $ | ( | $ | ( | $ | ||||||||||||||||||||||
See accompanying notes to consolidated financial statements.
67
MYRIAD GENETICS, INC.
AND SUBSIDIARIES
Consolidated Statements of Cash Flows
(in millions)
| Six-month Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| 2020 | 2020 | 2019 | 2018 | ||||||||||||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | |||||||||||||||||||||||
| Net income (loss) attributable to Myriad Genetics, Inc. stockholders | $ | ( | $ | ( | $ | $ | |||||||||||||||||
| Adjustments to reconcile net income (loss) to net cash provided by operating activities: | |||||||||||||||||||||||
| Depreciation and amortization | |||||||||||||||||||||||
| Non-cash interest expense | |||||||||||||||||||||||
| Non-cash lease expense | |||||||||||||||||||||||
| Gain on deconsolidation of subsidiary | ( | ||||||||||||||||||||||
| Non-cash impact of foreign currency transactions | ( | ||||||||||||||||||||||
| Gain on disposition of assets | ( | ( | ( | ||||||||||||||||||||
| Share-based compensation expense | |||||||||||||||||||||||
| Deferred income taxes | ( | ( | |||||||||||||||||||||
| Unrecognized tax benefits | ( | ( | |||||||||||||||||||||
| Impairment of goodwill and intangible assets | |||||||||||||||||||||||
| Change in fair value of contingent consideration | ( | ( | |||||||||||||||||||||
| Payment of contingent consideration | ( | ( | |||||||||||||||||||||
| Changes in assets and liabilities: | |||||||||||||||||||||||
| Prepaid expenses | ( | ||||||||||||||||||||||
| Trade accounts receivable | ( | ( | ( | ||||||||||||||||||||
| Other receivables | ( | ||||||||||||||||||||||
| Inventory | |||||||||||||||||||||||
| Prepaid taxes | ( | ( | |||||||||||||||||||||
| Other assets | ( | ||||||||||||||||||||||
| Accounts payable | ( | ( | |||||||||||||||||||||
| Accrued liabilities | ( | ||||||||||||||||||||||
| Deferred revenue | ( | ( | ( | ||||||||||||||||||||
| Net cash provided by (used in) operating activities | ( | ||||||||||||||||||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | |||||||||||||||||||||||
| Capital expenditures | ( | ( | ( | ( | |||||||||||||||||||
| Acquisitions, net of cash acquired | ( | ||||||||||||||||||||||
| Proceeds from sale of subsidiary | |||||||||||||||||||||||
| Purchases of marketable investment securities | ( | ( | ( | ||||||||||||||||||||
| Proceeds from maturities and sales of marketable investment securities | |||||||||||||||||||||||
| Net cash provided by (used in) investing activities | ( | ( | |||||||||||||||||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | |||||||||||||||||||||||
| Proceeds from common stock issued under share-based compensation plans | |||||||||||||||||||||||
| Payment of tax withheld for common stock issued under share-based compensation plans | ( | ( | ( | ( | |||||||||||||||||||
| Proceeds from revolving credit facility | |||||||||||||||||||||||
| Repayment of revolving credit facility | ( | ( | ( | ||||||||||||||||||||
| Payment of contingent consideration recognized at acquisition | ( | ( | ( | ||||||||||||||||||||
| Fees associated with refinancing of revolving credit facility | ( | ( | |||||||||||||||||||||
| Repurchase and retirement of common stock | ( | ||||||||||||||||||||||
| Proceeds from non-controlling interest | |||||||||||||||||||||||
| Net cash provided by (used in) financing activities | ( | ( | ( | ||||||||||||||||||||
| Effect of foreign exchange rates on cash and cash equivalents | ( | ||||||||||||||||||||||
| Net increase (decrease) in cash and cash equivalents | ( | ( | |||||||||||||||||||||
| Cash and cash equivalents at beginning of year | |||||||||||||||||||||||
| Cash and cash equivalents at end of year | $ | $ | $ | $ | |||||||||||||||||||
See accompanying notes to consolidated financial statements.
68
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(in millions, except per share data)
1. ORGANIZATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Financial Statement Presentation
Myriad Genetics, Inc. and subsidiaries (collectively, the "Company" or "Myriad") is a leading personalized precision medicine company acting as a trusted advisor to transform patient lives through molecular diagnostics. The Company employs a number of proprietary technologies, including DNA, RNA and protein analysis, that help it to understand the genetic basis of human disease and the role that genes and their related proteins may play in the onset and progression of disease. The Company uses this information to guide the development of molecular diagnostic and companion diagnostic tests that are designed to assess an individual’s risk for developing disease later in life (predictive medicine), identify a patient’s likelihood of responding to drug therapy and guide a patient’s dosing to ensure optimal treatment (personalized medicine), or assess a patient’s risk of disease progression and disease recurrence (prognostic medicine). The Company generates revenue by performing molecular diagnostic tests as well as by providing pharmaceutical and clinical services to the pharmaceutical and biotechnology industries and medical research institutions utilizing its multiplexed immunoassay technology. The Company’s corporate headquarters are located in Salt Lake City, Utah.
During the transition period ended December 31, 2020, the Company identified errors related to its accounting for its international subsidiaries and its determination of fair value of contingent consideration related to an acquisition that occurred in May 2016. These errors were not material to any previously reported period. Management analyzed the potential impact of these errors in accordance with the SEC Staff Accounting Bulletin No. 108, Considering the Effects of Prior Year Misstatements when Quantifying Misstatements in Current Year Financial Statements, and concluded that the correction of the errors are not material to the transition period ended December 31, 2020.
Use of Estimates
The preparation of the consolidated financial statements in accordance with U.S. GAAP requires Company management to make estimates and assumptions relating to the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the period. Significant items subject to such estimates and assumptions include revenue recognition estimates for the average expected reimbursement per test, valuation allowances for deferred income tax assets, certain accrued liabilities, share-based compensation, and impairment analysis of goodwill and intangible assets. Actual results could differ from those estimates.
The full impact of the COVID-19 outbreak continues to evolve and its future impacts remain uncertain and unpredictable. Management is actively monitoring the impact of the global situation on the Company’s financial condition, liquidity, operations, suppliers, industry, and workforce. Given the daily evolution of the COVID-19 outbreak and the global responses to curb its spread, the Company is not able to estimate the effects of the COVID-19 outbreak on its results of operations, financial condition, or liquidity for future periods.
69
Concentration of Credit Risk
Financial instruments that potentially subject us to concentrations of credit risk consist primarily of cash and cash equivalents and accounts receivable. Substantially all of the Company’s account receivable are with companies in the healthcare industry, U.S. and state governmental agencies, and individuals. The Company does not believe that receivables due from U.S. and state governmental agencies, such as Medicare, represent a credit risk since the related healthcare programs are funded by the U.S. and state governments. The Company only has one payor, Medicare, that represents greater than 10% of its revenues. Revenues received from Medicare are included within the Diagnostics reporting segment and represented approximately 16 %, 15 %, 14 % and 17 % of total revenue for the six-month transition period ended December 31, 2020 (the "Transition Period") and the fiscal years ended June 30, 2020, 2019 and 2018, respectively. Concentrations of credit risk are mitigated due to the number of the Company’s customers as well as their dispersion across many geographic regions. No customer accounted for more than 10% of accounts receivable at December 31, 2020, June 30, 2020 or June 30, 2019.
Marketable Investment Securities
The Company has classified its marketable investment securities, all of which are debt securities, as available-for-sale securities. These securities are carried at estimated fair value with unrealized holding gains and losses, net of the related tax effect, included in accumulated other comprehensive loss in stockholders’ equity until realized. Gains and losses on investment security transactions are reported on the specific-identification method. Dividend and interest income are recognized when earned. The Company’s cash equivalents consist of short-term, highly liquid investments that are readily convertible to known amounts of cash.
A decline in the market value of any available-for-sale security below cost that is deemed other than temporary results in a charge to earnings and establishes a new cost basis for the security. Losses are charged against Other income when a decline in fair value is determined to be other than temporary. The Company reviews several factors to determine whether a loss is other than temporary. These factors include but are not limited to: (i) the extent to which the fair value is less than cost and the cause for the fair value decline, (ii) the financial condition and near term prospects of the issuer, (iii) the length of time a security is in an unrealized loss position and (iv) the Company’s ability to hold the security for a period of time sufficient to allow for any anticipated recovery in fair value. There were no
Inventory
Inventories consist of supplies such as reagents, plates and testing kits, which are consumed when providing test results, and therefore the Company does not maintain finished goods inventory. Inventories are stated at the lower of cost or market and costs are determined on a first-in, first-out basis. In order to assess the ultimate realization of inventories, the Company is required to make judgments as to future demand requirements compared to current or committed inventory levels.
The Company evaluates its inventories for excess quantities and obsolescence. Inventories that are considered excess or obsolete are expensed. The valuation of inventories requires the use of estimates as to the amounts of current inventories that will be sold. These estimates are dependent on management’s assessment of current and expected orders from the Company’s customers.
Trade Accounts Receivable
Trade accounts receivable represents amounts billed to customers for revenue recognized related to molecular diagnostic tests and pharmaceutical and clinical services. The Company does not have any off-balance-sheet credit exposure related to its customers and does not require collateral.
Property, Plant and Equipment
Equipment and leasehold improvements are stated at cost less accumulated depreciation. Depreciation and amortization are computed using the straight-line method based on the lesser of estimated useful lives of the related assets or lease terms. Equipment items have depreciable lives of to seven years . Leasehold improvements are depreciated over the shorter of the estimated useful lives or the associated lease terms, which range from to seven years . Repairs and maintenance costs are charged to expense as incurred.
70
Intangible Assets and Other Long-Lived Assets
Intangible and other long-lived assets are comprised of acquired licenses, technology and intellectual property and purchased in-process research and development. Acquired intangible assets are recorded at fair value and amortized over the shorter of the contractual life or the estimated useful life. The estimated useful life of acquired in-process research and development was also evaluated in conjunction with the annual impairment analysis of intangible assets. The classification of the Company’s acquired in-process research and development as an indefinite lived asset was deemed appropriate as the related research and development was not yet complete nor had it been abandoned.
The Company capitalizes certain implementation costs incurred in a cloud computing arrangement to develop or obtain internal-use software, including hosting arrangements that include an internal-use software license. Implementation costs incurred in cloud computing arrangement are capitalized as part of Other assets in the Consolidated Balance Sheets.
The Company continually reviews and monitors long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future undiscounted cash flows, an impairment charge is recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
Goodwill
Goodwill is tested for impairment by reporting unit on an annual basis as of October 1 and in the interim if events and circumstances indicate that goodwill may be impaired. Prior to the change in the Company's fiscal year from the last day of June to a calendar fiscal year end, goodwill was tested for impairment on an annual basis as of April 1 and in the interim if events and circumstances indicated that goodwill may be impaired. The voluntary change is preferable under the circumstances as it provides the Company with additional time to complete its annual goodwill impairment testing in advance of its year-end reporting and results in better alignment with the Company’s strategic planning and forecasting process given the Company's change in fiscal year end. The events and circumstances that are considered include business climate and market conditions, legal factors, operating performance indicators and competition. Impairment of goodwill is first assessed using a qualitative approach. If the qualitative assessment suggests that impairment is more likely than not, a quantitative analysis is performed. The quantitative analysis involves a comparison of the fair value of the reporting unit with its carrying amount. If the carrying amount of a reporting unit exceeds its fair value, an impairment loss is recognized in an amount equal to that excess, limited to the total amount of goodwill allocated to that reporting unit. If an event occurs that would cause a revision to the estimates and assumptions used in analyzing the value of the goodwill, the revision could result in a non-cash impairment charge that could have a material impact on the financial results.
Revenue Recognition
Myriad generates revenue by performing molecular diagnostic testing and pharmaceutical services. The Company previously provided clinical services until selling Privatklinik Dr. Robert Schindlbeck GmbH & Co. KG (the "Clinic") in February 2020. Revenue from the sale of molecular diagnostic tests and pharmaceutical and clinical services is recorded at the estimated transaction price. The Company has determined that the communication of test results or the completion of clinical and pharmaceutical services indicates transfer of control for revenue recognition purposes.
71
The following table represents the Company’s revenue by type for the transition period ended December 31, 2020, and the fiscal years ended June 30, 2020, 2019, and 2018:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (In millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| Molecular diagnostic revenues: | |||||||||||||||||||||||
| Hereditary Cancer Testing | $ | $ | $ | $ | |||||||||||||||||||
| GeneSight | |||||||||||||||||||||||
| Prenatal | |||||||||||||||||||||||
| Vectra | |||||||||||||||||||||||
| myChoice CDx | |||||||||||||||||||||||
| Prolaris | |||||||||||||||||||||||
| EndoPredict | |||||||||||||||||||||||
| Other | |||||||||||||||||||||||
| Total molecular diagnostic revenue | |||||||||||||||||||||||
| Pharmaceutical and clinical service revenue | |||||||||||||||||||||||
| Total revenue | $ | $ | $ | $ | |||||||||||||||||||
Under ASC Topic 606, Revenue from Contracts with Customers (“Topic 606”), an entity recognizes revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The Company performs its obligation under a contract with a customer by processing diagnostic tests and communicating the test results to customers, in exchange for consideration from the customer. The Company has the right to bill its customers upon the completion of performance obligations and thus does not record contract assets. Occasionally customers make payments prior to the Company's performance of its contractual obligations. When this occurs, the Company records a contract liability as deferred revenue. During the fiscal year ended June 30, 2020, the Company received approximately $29.7 million in advance Medicare payments as part of the CARES Act, which was enacted on March 27, 2020 to provide relief from the economic impacts of COVID-19. Repayment of the advanced Medicare payments begins April 2021. The advance Medicare payments are included in prepayments of deferred revenue for the fiscal year ended June 30, 2020 and in the beginning and ending balance of deferred revenue for the transition period ended December 31, 2020. A reconciliation of the beginning and ending balances of deferred revenue is shown in the table below:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | ||||||||||||||
| Deferred revenue - beginning balance | $ | $ | $ | ||||||||||||||
| Revenue recognized | ( | ( | ( | ||||||||||||||
| Prepayments | |||||||||||||||||
| Deferred revenue - ending balance | $ | $ | $ | ||||||||||||||
In accordance with Topic 606, the Company has elected not to disclose the aggregate amount of the transaction price allocated to remaining performance obligations for its contracts that are one year or less, as the revenue is expected to be recognized within the next year. Furthermore, the Company has elected not to disclose the aggregate amount of the transaction price allocated to remaining performance obligations for its agreements wherein the Company’s right to payment is in an amount that directly corresponds with the value of Company’s performance to date. However, periodically, the Company enters into arrangements with customers to provide diagnostic testing and/or pharmaceutical and clinical services that may have terms longer than one year and include multiple performance obligations. As of December 31, 2020, the aggregate amount of the transaction price of such contracts that is allocated to the remaining performance obligations is $11.2 million.
72
In determining the transaction price, Myriad includes an estimate of the expected amount of consideration as revenue. The Company applies this method consistently for similar contracts when estimating the effect of any uncertainty on an amount of variable consideration to which it will be entitled. An estimate of transaction price does not include any estimated amount of variable consideration that are constrained. In addition, the Company considers all the information (historical, current, and forecast) that is reasonably available to identify possible consideration amounts. In determining the expected value, the Company considers the probability of the variable consideration for each possible scenario. The Company also has significant experience with historical discount patterns and uses this experience to estimate transaction prices.
The estimate of revenue is affected by assumptions in payor behavior such as changes in payor mix, payor collections, current customer contractual requirements, and experience with collections from third-party payors. When assessing the total consideration for insurance carriers and patients, revenues are further constrained for estimated refunds. The Company reserves certain amounts in Accrued liabilities in the Consolidated Balance Sheets in anticipation of request for refunds of payments made previously by insurance carriers, which are accounted for as reductions in revenues in the Consolidated Statements of Operations and Comprehensive Income (Loss).
Cash collections for certain diagnostic tests delivered may differ from rates originally estimated, primarily driven by changes in the estimated transaction price due to contractual adjustments, obtaining updated information from payors and patients that was unknown at the time the performance obligation was met and settlements with third party payors. As a result of this new information, the Company updates its estimate of the amounts to be recognized for previously delivered tests. During the transition period ended December 31, 2020, and fiscal year ended June 30, 2019 and 2018, the impact to revenue and earnings (loss) per share, for tests in which the performance obligation of delivering the test results was met in the prior period was immaterial. During the fiscal year ended June 30, 2020, the Company recognized a $9.9 million decrease in revenue, which resulted in a $(0.10 ) impact to earnings (loss) per share for tests in which the performance obligation of delivering the tests results was met in prior periods. The changes were primarily driven by changes in the estimated transaction price due to contractual adjustments, obtaining updated information from payors and patients that was unknown at the time the performance obligation was met and settlements with third-party payors. In addition, during the fiscal year ended June 30, 2020, the Company identified an error related to prior periods for Medicare claims and has reduced revenue and recorded an accrued liability for a total of $4.7 0.05 ).
In accordance with Topic 606, the Company has elected to exclude from the measurement of transaction price, all taxes assessed by a governmental authority that are both imposed on and concurrent with a specific revenue-producing transaction and collected by the Company from a customer for e.g. sales tax, value added tax etc.
The Company has elected to apply the practical expedient related to costs to obtain or fulfill a contract since the amortization period for such costs will be one year or less. Accordingly, no costs incurred to obtain or fulfill a contract have been capitalized. The Company has also elected to apply the practical expedient for not adjusting revenue recognized for the effects of the time value of money. This practical expedient has been elected because the Company collects very little cash from customers under payment terms and vast majority of payments terms have a payback period of less than one year.
Share-based payment expense
We recognize the fair value compensation cost relating to share-based payment transactions in accordance with Accounting Standards Codification (“ASC”) 718, Compensation – Stock Compensation. Under the provisions of ASC 718, stock-based compensation cost is measured at the grant date, based on the fair value of the award, and is recognized over the employee’s requisite service period, which is generally the vesting period. The fair value of restricted stock units (RSUs) is based on the number of shares granted and the quoted price of the Company’s common stock on the grant date. Forfeitures are recognized as a reduction of compensation expense in earnings in the period in which they occur. The fair value of shares issued under the Employee Stock Purchase Plan is calculated using the Black-Scholes option-pricing model, based on assumptions including the risk-free interest rate, expected life, expected dividend yield and expected volatility. The average risk-free interest rate is determined using the U.S. Treasury rate. We determine the expected life based on offering period of the Employee Stock Purchase Plan. The expected volatility is determined using the weighted average of daily historical volatility of our stock price.
73
Other Income
The Company recognizes stimulus or grant payments that it receives that do not need to be paid back as other income. During the fiscal year ended June 30, 2020, the Company received approximately $14.6 million from the Provider Relief Fund under the CARES Act to reimburse the Company for health care related expenses or lost revenues that are attributable to COVID-19, which was recognized as a component of Other income in the Consolidated Statements of Operations.
Income Taxes
The Company recognizes income taxes under the asset and liability method. This approach requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of assets and liabilities.
The provision for income taxes, including the effective tax rate and analysis of potential tax exposure items, if any, requires significant judgment and expertise in federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and any estimated valuation allowances deemed necessary to recognize deferred tax assets at an amount that is more likely than not to be realized. The Company’s filings, including the positions taken therein, are subject to audit by various taxing authorities. While the Company believes it has provided adequately for its income tax liabilities in the consolidated financial statements, adverse determinations by these taxing authorities could have a material adverse effect on the consolidated financial condition, results of operations or cash flows.
Earnings Per Share
Basic earnings per share is computed based on the weighted-average number of shares of common stock outstanding. Diluted earnings per share is computed based on the weighted-average number of shares of common stock, including the dilutive effect of common stock equivalents, outstanding.
The following is a reconciliation of the denominators of the basic and diluted earnings per share computations:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| Denominator: | |||||||||||||||||||||||
| Weighted-average shares outstanding used to compute basic EPS | |||||||||||||||||||||||
| Effect of dilutive stock options | |||||||||||||||||||||||
| Weighted-average shares outstanding and dilutive securities used to compute diluted EPS | |||||||||||||||||||||||
Certain outstanding options and RSUs were excluded from the computation of diluted earnings per share because the effect would have been anti-dilutive. These potential dilutive common shares, which may be dilutive to future diluted earnings per share, are as follows:
| Transition Period Ended December 31, | Years Ending June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
Anti-dilutive options and RSUs excluded from EPS computation | |||||||||||||||||||||||
74
Foreign Currency
The functional currency of the Company’s international subsidiaries is the local currency. For those subsidiaries, expenses denominated in the functional currency are translated into U.S. dollars using average exchange rates in effect during the period and assets and liabilities are translated using period-end exchange rates. The foreign currency translation adjustments are included in Accumulated other comprehensive income (loss) as a separate component of Stockholders’ equity.
The following table shows the cumulative translation adjustments included in Accumulated other comprehensive income (loss) (in millions):
| Ending balance June 30, 2020 | $ | ( | |||
| Period translation adjustments | |||||
| Other | |||||
| Ending balance December 31, 2020 | $ | ( | |||
Reclassifications
Certain prior period amounts have been reclassified to conform with the current period presentation. The reclassifications have no impact on the total assets, total liabilities, stockholders' equity, cash flows from operations, or net loss for the period.
Recent Accounting Pronouncements
Standard Effective in Future Years and Not Yet Adopted
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes ("ASU 2019-12"). ASC 2019-12 is a new accounting standard to simplify accounting for income taxes and remove, modify, and add to the disclosure requirements of income taxes. The standard is effective for public companies with fiscal years beginning after December 15, 2020, with early adoption permitted. The Company does not expect the adoption of this standard to have a material impact on its consolidated financial statements.
Recently Adopted Standards
In June 2016, the FASB issued ASU 2016-13, Financial Instruments – Credit Losses (Topic 326) (“ASU 2016-13”) which introduces new guidance for the accounting for credit losses on certain instruments within its scope. ASU 2016-13 introduces an approach based on expected losses to estimate credit losses on certain types of financial instruments. For trade receivables, the Company will be required to use a forward-looking expected loss model rather than the incurred loss model for recognizing credit losses, which reflects losses that are probable. Credit losses relating to available-for-sale debt securities will also be recorded through an allowance for credit losses rather than as a reduction in the amortized cost basis of the securities. On July 1, 2020, the Company adopted ASU 2016-13 under the modified retrospective approach by initially applying ASU 2016-13 at the adoption date, rather than at the beginning of the earliest comparative period presented. This guidance was adopted with no material impact to the Company's consolidated financial statements.
In August 2018, the FASB issued ASU 2018-15, Intangibles – Goodwill and Other - Internal-Use Software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract (“ASU 2018-15”). ASU 2018-15 aligns the requirements for capitalizing implementation costs incurred in a hosting arrangement that is a service contract with the requirements for capitalizing implementation costs incurred to develop or obtain internal-use software, including hosting arrangements that include an internal-use software license. On July 1, 2020, the Company adopted ASU 2018-15 on a prospective basis with no material impact to the Company's consolidated financial statements. The amounts capitalized may be material in future periods; implementation costs incurred in cloud computing arrangements are capitalized as part of Other assets in the Consolidated Balance Sheets.
75
2. MARKETABLE INVESTMENT SECURITIES
The amortized cost, gross unrealized holding gains, gross unrealized holding losses, and fair value debt securities classified as available-for-sale securities by major security type and class of security at December 31, 2020, June 30, 2020 and June 30, 2019 were as follows:
| (in millions) | Amortized cost | Gross unrealized holding gains | Gross unrealized holding losses | Estimated fair value | |||||||||||||||||||
| December 31, 2020: | |||||||||||||||||||||||
| Cash and cash equivalents: | |||||||||||||||||||||||
| Cash | $ | $ | — | $ | — | $ | |||||||||||||||||
| Cash equivalents | — | — | |||||||||||||||||||||
| Total cash and cash equivalents | — | — | |||||||||||||||||||||
| Available-for-sale: | |||||||||||||||||||||||
| Corporate bonds and notes | |||||||||||||||||||||||
| Municipal bonds | |||||||||||||||||||||||
| Federal agency issues | |||||||||||||||||||||||
| US government securities | |||||||||||||||||||||||
| Total | $ | $ | $ | $ | |||||||||||||||||||
| (in millions) | Amortized cost | Gross unrealized holding gains | Gross unrealized holding losses | Estimated fair value | |||||||||||||||||||
| June 30, 2020: | |||||||||||||||||||||||
| Cash and cash equivalents: | |||||||||||||||||||||||
| Cash | $ | $ | — | $ | — | $ | |||||||||||||||||
| Cash equivalents | — | — | |||||||||||||||||||||
| Total cash and cash equivalents | — | — | |||||||||||||||||||||
| Available-for-sale: | |||||||||||||||||||||||
| Corporate bonds and notes | |||||||||||||||||||||||
| Municipal bonds | |||||||||||||||||||||||
| Federal agency issues | |||||||||||||||||||||||
| US government securities | |||||||||||||||||||||||
| Total | $ | $ | $ | $ | |||||||||||||||||||
76
| (in millions) | Amortized cost | Gross unrealized holding gains | Gross unrealized holding losses | Estimated fair value | |||||||||||||||||||
| June 30, 2019: | |||||||||||||||||||||||
| Cash and cash equivalents: | |||||||||||||||||||||||
| Cash | $ | $ | — | $ | — | $ | |||||||||||||||||
| Cash equivalents | — | — | |||||||||||||||||||||
| Total cash and cash equivalents | — | — | |||||||||||||||||||||
| Available-for-sale: | |||||||||||||||||||||||
| Corporate bonds and notes | |||||||||||||||||||||||
| Municipal bonds | |||||||||||||||||||||||
| Federal agency issues | |||||||||||||||||||||||
| US government securities | |||||||||||||||||||||||
| Total | $ | $ | $ | $ | |||||||||||||||||||
Cash, cash equivalents, and maturities of debt securities classified as available-for-sale are as follows at December 31, 2020:
| (in millions) | Amortized cost | Estimated fair value | |||||||||
| Cash | |||||||||||
| Cash equivalents | |||||||||||
| Available-for-sale: | |||||||||||
| Due within one year | |||||||||||
| Due after one year through five years | |||||||||||
| Due after five years | |||||||||||
| Total | $ | $ | |||||||||
There were no
Additional information relating to fair value of marketable investment securities can be found in Note 3.
3. FAIR VALUE MEASUREMENTS
The fair value of the Company’s financial instruments reflects the amounts that the Company estimates to receive in connection with the sale of an asset or paid in connection with the transfer of a liability in an orderly transaction between market participants at the measurement date (exit price). The fair value hierarchy prioritizes the use of inputs used in valuation techniques into the following three levels:
Level 1—quoted prices in active markets for identical assets and liabilities.
Level 2— observable inputs other than quoted prices in active markets for identical assets and liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. Some of the Company’s marketable securities primarily utilize broker quotes in a non-active market for valuation of these securities.
Level 3—unobservable inputs.
77
All of the Company’s financial instruments are valued using quoted prices in active markets or based on other observable inputs. For Level 2 securities, the Company uses a third-party pricing service which provides documentation on an ongoing basis that includes, among other things, pricing information with respect to reference data, methodology, inputs summarized by asset class, pricing application and corroborative information. For Level 3 contingent consideration, the Company reassesses the fair value of expected contingent consideration and the corresponding liability each reporting period using the Monte Carlo Method, which is consistent with the initial measurement of the expected earn out liability. This fair value measurement is considered a Level 3 measurement because the Company estimates projections during the earn out period of approximately 14.5 years utilizing various potential pay-out scenarios. Probabilities were applied to each potential scenario and the resulting values were discounted using a rate that considers weighted average cost of capital as well as a specific risk premium associated with the riskiness of the earn out itself, the related projections, and the overall business. The contingent consideration liabilities are classified as a component of long-term and short-term contingent consideration in the Company’s Consolidated Balance Sheets. Changes to the contingent consideration liabilities are reflected in Change in the fair value of contingent consideration in our Consolidated Statements of Operations. Changes to the unobservable inputs could have a material impact on the Company’s financial statements.
The fair value of our long-term debt, which we consider a Level 3 measurement, is estimated using discounted cash flow analyses, based on the Company’s current estimated incremental borrowing rates for similar borrowing arrangements. The fair value of long-term debt is estimated to be $225.8 million at December 31, 2020, $225.5 million at June 30, 2020 and $192.7 million at June 30, 2019.
The following tables set forth the fair value of the Company’s financial assets and liabilities that are re-measured on a regular basis:
| (in millions) | Level 1 | Level 2 | Level 3 | Total | |||||||||||||||||||
| December 31, 2020 | |||||||||||||||||||||||
| Money market funds (a) | $ | $ | $ | $ | |||||||||||||||||||
| Corporate bonds and notes | |||||||||||||||||||||||
| Municipal bonds | |||||||||||||||||||||||
| Federal agency issues | |||||||||||||||||||||||
| US government securities | |||||||||||||||||||||||
| Contingent consideration | ( | ( | |||||||||||||||||||||
| Total | $ | $ | $ | ( | $ | ||||||||||||||||||
(a)Money market funds are primarily comprised of exchange traded funds and accrued interest.
| (in millions) | Level 1 | Level 2 | Level 3 | Total | |||||||||||||||||||
| June 30, 2020 | |||||||||||||||||||||||
| Money market funds (a) | $ | $ | $ | $ | |||||||||||||||||||
| Corporate bonds and notes | |||||||||||||||||||||||
| Municipal bonds | |||||||||||||||||||||||
| Federal agency issues | |||||||||||||||||||||||
| US government securities | |||||||||||||||||||||||
| Contingent consideration | ( | ( | |||||||||||||||||||||
| Total | $ | $ | $ | ( | $ | ||||||||||||||||||
(a)Money market funds are primarily comprised of exchange traded funds and accrued interest.
78
| (in millions) | Level 1 | Level 2 | Level 3 | Total | |||||||||||||||||||
| June 30, 2019 | |||||||||||||||||||||||
| Money market funds (a) | $ | $ | $ | $ | |||||||||||||||||||
| Corporate bonds and notes | |||||||||||||||||||||||
| Municipal bonds | |||||||||||||||||||||||
| Federal agency issues | |||||||||||||||||||||||
| US government securities | |||||||||||||||||||||||
| Contingent consideration | ( | ( | |||||||||||||||||||||
| Total | $ | $ | $ | ( | $ | ||||||||||||||||||
(a)Money market funds are primarily comprised of exchange traded funds and accrued interest.
The following table reconciles the change in the fair value of the contingent consideration during the periods presented:
| (in millions) | Transition Period Ended December 31, 2020 | Year Ended June 30, 2020 | Year Ended June 30, 2019 | ||||||||||||||
| Carrying amount at beginning of period | $ | $ | $ | ||||||||||||||
| Payment of contingent consideration | ( | ( | |||||||||||||||
| Change in fair value recognized in the statement of operations | ( | ( | |||||||||||||||
| Translation adjustments recognized in other comprehensive income | ( | ||||||||||||||||
| Carrying amount at end of period | $ | $ | $ | ||||||||||||||
4. PROPERTY, PLANT AND EQUIPMENT, NET
| December 31, | June 30, | ||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | ||||||||||||||
| Land | $ | $ | $ | ||||||||||||||
| Buildings and improvements | |||||||||||||||||
| Leasehold improvements | |||||||||||||||||
| Equipment | |||||||||||||||||
| Property, plant and equipment, gross | |||||||||||||||||
| Less accumulated depreciation | ( | ( | ( | ||||||||||||||
| Property, plant and equipment, net | $ | $ | $ | ||||||||||||||
During the fiscal year ended June 30, 2020, the Company sold the Clinic resulting in the deconsolidation of $19.5 million of the balance of property, plant and equipment. See Note 16 for additional information regarding the sale of the Clinic.
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| Depreciation expense | $ | $ | $ | $ | |||||||||||||||||||
79
5. GOODWILL AND INTANGIBLE ASSETS
Goodwill
The changes in the carrying amount of goodwill for the transition period ended December 31, 2020 are as follows:
| (in millions) | Diagnostic | Other | Total | ||||||||||||||
| Beginning balance | $ | $ | $ | ||||||||||||||
| Translation adjustments | |||||||||||||||||
| Ending balance | $ | $ | $ | ||||||||||||||
The changes in the carrying amount of goodwill for the fiscal years ended June 30, 2020 and 2019 are as follows:
| Diagnostic | Other | Total | |||||||||||||||||||||||||||||||||
| Years Ended June 30, | Years Ended June 30, | Years Ended June 30, | |||||||||||||||||||||||||||||||||
| (in millions) | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | |||||||||||||||||||||||||||||
| Beginning balance | $ | $ | $ | $ | $ | $ | |||||||||||||||||||||||||||||
| Acquisitions (see note 17) | |||||||||||||||||||||||||||||||||||
| Adjustments to acquisitions (see note 17) | |||||||||||||||||||||||||||||||||||
Goodwill deconsolidated on sale of Clinic | ( | ( | |||||||||||||||||||||||||||||||||
| Goodwill impairment charge | ( | ( | ( | ||||||||||||||||||||||||||||||||
| Translation adjustments | ( | ( | ( | ( | ( | ( | |||||||||||||||||||||||||||||
| Ending balance | $ | $ | $ | $ | $ | $ | |||||||||||||||||||||||||||||
The Company assessed goodwill for impairment in accordance with the appropriate guidance (see Note 1) and determined no ne of its reporting units were impaired during the transition period ended December 31, 2020.
During the fiscal year ended June 30, 2020, as a result of the effect of COVID-19 on expected future cash flows and a corresponding decline in market capitalization and enterprise value, the Company performed an interim quantitative impairment review of goodwill for the Myriad Mental Health, Myriad Autoimmune and Myriad International reporting units as of March 31, 2020. Based on this analysis, the Company recognized a goodwill impairment charge of $80.7 million related to the goodwill from the Myriad Autoimmune reporting unit. The Myriad Autoimmune reporting unit is part of the Company’s diagnostic segment. The goodwill impairment charge is reflected in Goodwill and intangible asset impairment charges in the Consolidated Statements of Operations.
During the fiscal year ended June 30, 2020, the Company also recognized a $1.3 million impairment charge for goodwill allocated to the Clinic asset group that is included in Goodwill and intangible asset impairment charges in the Consolidated Statements of Operations. The Clinic asset group was part of the Company’s other segment.
Intangible assets primarily consist of amortizable assets of purchased licenses and technologies, developed technology, a laboratory database, trademarks, and customer relationships as well as a non-amortizable intangible asset of in-process research and development. The Company’s developed technology and database acquired have estimated remaining useful lives between 1 and 16 years, trademarks acquired have an estimated remaining useful life of approximately eight years and customer relationships have an estimated remaining useful life of approximately one year . The estimated useful life of acquired in-process research and development was also evaluated in conjunction with the annual impairment analysis of intangible assets. The classification of the acquired in-process research and development as an indefinite lived asset was deemed appropriate as the related research and development was not yet complete nor had it been abandoned. During the fiscal year ended June 30, 2020, the Company decided to abandon the development of one of its in-process research and development intangible assets, and as a result the Company recognized a charge of $17.7 million, which is reflected in Goodwill and intangible asset impairment charges in the Consolidated Statements of Operations. The abandoned in-process research and development intangible asset was reported as part of the Company’s diagnostic segment. The Company concluded there was no
80
The following tables summarize the amounts reported as intangible assets (in millions):
| At December 31, 2020: | Gross Carrying Amount | Accumulated Amortization | Net | ||||||||||||||
| Purchased licenses and technologies | $ | $ | ( | $ | |||||||||||||
| Customer relationships | ( | ||||||||||||||||
| Trademarks | ( | ||||||||||||||||
| Total amortizable intangible assets | ( | ||||||||||||||||
| In-process research and development | — | ||||||||||||||||
| Total unamortized intangible assets | — | ||||||||||||||||
| Total intangible assets | $ | $ | ( | $ | |||||||||||||
| At June 30, 2020: | Gross Carrying Amount | Accumulated Amortization | Net | ||||||||||||||
| Purchased licenses and technologies | $ | $ | ( | $ | |||||||||||||
| Customer relationships | ( | ||||||||||||||||
| Trademarks | ( | ||||||||||||||||
| Total amortizable intangible assets | ( | ||||||||||||||||
| In-process research and development | — | ||||||||||||||||
| Total unamortized intangible assets | — | ||||||||||||||||
| Total intangible assets | $ | $ | ( | $ | |||||||||||||
| At June 30, 2019: | Gross Carrying Amount | Accumulated Amortization | Net | ||||||||||||||
| Purchased licenses and technologies | $ | $ | ( | $ | |||||||||||||
| Customer relationships | ( | ||||||||||||||||
| Trademarks | ( | ||||||||||||||||
| Total amortizable intangible assets | ( | ||||||||||||||||
| In-process research and development | — | ||||||||||||||||
| Total unamortized intangible assets | — | ||||||||||||||||
| Total intangible assets | $ | $ | ( | $ | |||||||||||||
As of December 31, 2020 the weighted average remaining amortization period for purchased licenses and technologies, trademarks, and customer relationships is approximately 11 years.
The Company recorded amortization during the respective periods for these intangible assets as follows:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| Amortization of intangible assets | $ | $ | $ | $ | |||||||||||||||||||
81
Future amortization expense of intangible assets as of December 31, 2020 is estimated to be as follows (in millions):
| Years Ended December 31, | Amortization Expense | ||||
| 2021 | $ | ||||
| 2022 | |||||
| 2023 | |||||
| 2024 | |||||
| 2025 | |||||
| Thereafter | |||||
| Total | $ | ||||
6. ACCRUED LIABILITIES
| December 31, | June 30, | ||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | ||||||||||||||
| Employee compensation and benefits | $ | $ | $ | ||||||||||||||
| Accrued taxes payable | |||||||||||||||||
| Qui tam settlement | |||||||||||||||||
| Recoupments payable and reserves | |||||||||||||||||
Short-term contingent consideration | |||||||||||||||||
| Other | |||||||||||||||||
| Total accrued liabilities | $ | $ | $ | ||||||||||||||
7. LONG-TERM DEBT
On December 23, 2016, the Company entered into a senior secured revolving credit facility (the “Facility”) as borrower, with the lenders from time to time party thereto. On July 31, 2018, the Company entered into Amendment No. 1 to the Facility (the “Amended Facility”), which effected an “amend and extend” transaction with respect to the Facility by which the maturity date thereof was extended to July 31, 2023 and the maximum aggregate principal commitment was increased from $300.0 million to $350.0 million. On May 1, 2020, the Company entered into Amendment No. 2 to the Amended Facility, which waived the Company’s compliance with certain covenants and modified the interest rate and other terms during the Amendment Period from March 31, 2020 through June 30, 2021 (“Amendment Period”). Both amendments were accounted for as modifications pursuant to guidance in ASC 470-50.
Pursuant to the Amended Facility, the Company borrowed revolving loans in an aggregate principal amount of $300.0 million with $1.8 million in upfront fees and $0.3 million debt issuance costs recorded as a debt discount to be amortized over the term of the Amended Facility. The Company incurred an additional $1.0 million in upfront fees as a result of Amendment No. 2, which was also recorded as a debt discount that will be amortized over the term of the Amended Facility. There are no scheduled principal payments of the Amended Facility prior to its maturity date.
The proceeds of the Amended Facility were used to: (i) refinance in full the obligations under the Facility, (ii) finance the acquisition of Counsyl (See Note 17), (iii) pay fees, commissions, transactions costs and expenses incurred in connection with the foregoing, and (iv) for working capital and other general corporate purposes.
The Amended Facility contains customary loan terms, interest rates, representations and warranties, affirmative and negative covenants, in each case, subject to customary limitations, exceptions and exclusions. The Amended Facility also contains certain customary events of default. Amendment No. 2 modified the Amended Facility to increase the interest rate to be fixed at a spread of LIBOR plus 350 basis points on drawn balances and the undrawn fee was increased to 50 basis points during the Amendment Period, at which point they return to the existing pricing of 200 basis points on drawn balances and an undrawn fee ranging from 25 to 45 basis points based on the Company’s leverage ratio. The LIBOR floor was also increased to 1.0 % during the Amendment Period. The interest rate as of December 31, 2020 was 4.5 %.
82
Covenants in the Amended Facility impose operating and financial restrictions on the Company. These restrictions may prohibit or place limitations on, among other things, the Company’s ability to incur additional indebtedness, create certain types of liens or complete mergers or consolidations, and/or change in control transactions. The Amended Facility may also prohibit or place limitations on the Company’s ability to sell assets, pay dividends or provide other distributions to stockholders. The Company must maintain specified leverage and interest ratios measured as of the end of each quarter as a financial covenant in the Amended Facility. Amendment No. 2 modified the Amended Facility’s compliance with the leverage ratio covenant and the interest coverage ratio covenant, which were waived through March 31, 2021. A minimum liquidity covenant was added for the period beginning May 2020 until March 2021, and a minimum EBITDA covenant was added for the quarters ending December 31, 2020 and March 31, 2021. Amendment No. 2 also revised certain negative covenants of the Amended Facility during the Amendment Period. As of December 31, 2020, the Company was not in compliance with the minimum EBITDA covenant related to the Amended Facility. The Company is in compliance with all debt covenants as of the date the consolidated financial statements are available for issuance. As discussed in Note 18, on February 22, 2021 the Company entered into Amendment No. 3 to the Amended Facility which removed the minimum EBITDA covenant and also amended other covenant terms.
During the transition period ended December 31, 2020, the Company did no t make any principal repayments. During the fiscal years ended June 30, 2020, 2019 and 2018 the Company made $8.6 million, $115.0 million and $143.0 million in principal repayments, respectively.
The Amended Facility is secured by a first-lien security interest in substantially all of the assets of Myriad and certain of its domestic subsidiaries and each such domestic subsidiary of Myriad has guaranteed the repayment of the Amended Facility. Amounts outstanding under the Amended Facility were as follows:
| December 31, | June 30, | ||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | ||||||||||||||
| Long-term debt | $ | $ | $ | ||||||||||||||
| Long-term debt discount | ( | ( | ( | ||||||||||||||
| Net long-term debt | $ | $ | $ | ||||||||||||||
8. OTHER LONG-TERM LIABILITIES
| December 31, | June 30, | ||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | ||||||||||||||
| Pension obligation | $ | $ | $ | ||||||||||||||
| Contingent consideration | |||||||||||||||||
| Other | |||||||||||||||||
| Total other long-term liabilities | $ | $ | $ | ||||||||||||||
The Company’s balance of other long-term liabilities for the transition period ended December 31, 2020 consists of Company’s portion of social security taxes that have been deferred under the CARES Act that do not have to be deposited until December 2022. The Company previously held two non-contributory defined benefit pension plans for its current and former Clinic employees. The Company has closed participation in the plans to exclude those employees hired after 2002. As of June 30, 2019 the fair value of the plan assets were approximately $0.1 million resulting in a net pension liability of $6.8 million. The Company sold the Clinic in February 2020 and as a result the net pension liability was removed upon deconsolidation. See Note 16 for further discussion regarding the sale of the Clinic.
83
9. PREFERRED AND COMMON STOCKHOLDERS' EQUITY
The Company is authorized to issue up to 5.0 shares of preferred stock, par value $0.01 per share. There were no
The Company is authorized to issue up to 150.0 million shares of common stock, par value $0.01 per share. There were 75.4 74.7 73.5 70.6
Common shares issued and outstanding
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| Beginning common stock issued and outstanding | |||||||||||||||||||||||
Common stock issued upon exercise of options and employee stock plans | |||||||||||||||||||||||
| Repurchase and retirement of common stock | ( | ||||||||||||||||||||||
| Ending common stock issued and outstanding | |||||||||||||||||||||||
Stock Repurchase Program
In June 2016, the Company’s Board of Directors authorized a share repurchase program of $200.0 million of the Company’s outstanding common stock. The Company may repurchase its common stock from time to time or on an accelerated basis through open market transactions or privately negotiated transactions as determined by the Company’s management. The amount and timing of stock repurchases under the program will depend on business and market conditions, stock price, trading restrictions, acquisition activity and other factors. As of December 31, 2020, the Company has $110.7 million remaining on its current share repurchase authorization.
The Company uses the par value method of accounting for its stock repurchases. As a result of the stock repurchases, the Company reduced common stock and additional paid-in capital and recorded charges to Retained earnings (accumulated deficit). During the fiscal year ended June 30, 2019 the Company used $50.0 million to repurchase shares of the Company’s stock as part of an accelerated share repurchase. The shares retired, aggregate common stock and additional paid-in capital reductions, and related charges to Retained earnings (accumulated deficit) for the repurchases for periods ended December 31, 2020, June 30, 2020, June 30, 2019, and June 30, 2018 were as follows:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| Shares purchased and retired | |||||||||||||||||||||||
| Common stock and additional paid-in-capital reductions | $ | $ | $ | $ | |||||||||||||||||||
| Charges to retained earnings | $ | $ | $ | $ | |||||||||||||||||||
10. SHARE-BASED COMPENSATION
84
The number of shares, terms, and vesting periods are determined by the Company’s Board of Directors or a committee thereof on an award-by-award basis. RSUs granted to employees generally vest ratably over four years from the month of the anniversary date in which the RSUs are granted. Options and RSUs granted to our non-employee directors vest in full upon completion of one year of service on the anniversary following the date of the grant. Options generally vest ratably over service periods of four years . Options granted after December 5, 2012 expire eight years from the date of grant, and options granted prior to that date generally expire ten years from the date of grant. In September 2014, the Company began generally issuing RSUs in lieu of stock options.
The number of RSUs awarded to certain executive officers that ultimately vest may be increased or reduced based on certain additional performance and market conditions. The performance and market conditions associated with awards granted during the transition period ended December 31, 2020 include vesting that is based 50 % on achieving certain levels of earnings per share targets, and 50 % based on achieving certain performance targets compared to the performance of the Nasdaq Healthcare Provider Index. The Company estimates the likelihood of achievement of performance conditions at the end of each period.
During the transition period ended December 31, 2020, the Company granted stock-based awards to the Company's President and Chief Executive Officer as an inducement material to his commencement of employment and entry into an employment agreement with the Company. The awards that were granted consist of time-based RSUs as well as RSUs that have the same performance conditions as other executives discussed in the preceding paragraph. The awards also consist of time-based stock options as well as stock options with market-based vesting conditions based on achievement of certain levels of Myriad's stock price for a period of 20 consecutive days. The inducement awards were made in accordance with Nasdaq Stock Market rules and were not made under the Company's existing equity plans. The inducement awards are included in the tables presented.
Stock Options
A summary of option activity under the Company's equity plans, including the Company's inducement awards, is as follows for the transition period ended December 31, 2020:
| (number of shares in millions) | Number of shares | Weighted average exercise price | |||||||||
| Options outstanding at beginning of period | $ | ||||||||||
| Options granted | |||||||||||
| Less: | |||||||||||
| Options exercised | |||||||||||
| Options canceled or expired | ( | ||||||||||
| Options outstanding at end of period | |||||||||||
| Options exercisable at end of period | |||||||||||
| Options vested and expected to vest | |||||||||||
The fair value of options granted is estimated on the grant date using the Black-Scholes option-pricing model except for options with a market condition, which are valued using a Monte Carlo Simulation. The fair value of the stock options is amortized on a straight-line basis over the requisite service period of the awards, which is generally the vesting period. During the transition period ended December 31, 2020, the valuation assumptions used for stock options were as follows:
| Transition Period Ended December 31, | |||||
| 2020 | |||||
| Risk-free interest rate | |||||
| Expected dividend yield | |||||
| Expected life (in years) | |||||
| Derived service period (in years) | |||||
| Expected volatility | |||||
85
There were no
The following table summarizes information about stock options outstanding at December 31, 2020 (number of shares in millions):
| Options outstanding and exercisable | ||||||||||||||||||||
| Range of exercise prices | Number outstanding at December 31, 2020 | Weighted average remaining contractual life (years) | Weighted average exercise price | |||||||||||||||||
| $ | ||||||||||||||||||||
| $ | ||||||||||||||||||||
As of December 31, 2020 there was $4.6 million of unrecognized share-based compensation expense related to the inducement stock options that will be recognized over a weighted-average period of 2.5 years.
Restricted Stock Units
A summary of the RSU activity under the Company's equity plans, including the Company's inducement awards and RSU awards with performance metrics, is as follows for the transition period ended December 31, 2020:
| 2020 | |||||||||||
| (number of shares in millions) | Number of shares | Weighted average grant date fair value | |||||||||
| RSUs outstanding at beginning of period | $ | ||||||||||
| RSUs granted | |||||||||||
| Less: | |||||||||||
| RSUs vested | ( | ||||||||||
| RSUs canceled | ( | ||||||||||
| RSUs outstanding at end of period | $ | ||||||||||
The weighted average grant-date fair value of restricted stock units grants during the transition period ended December 31, 2020, and the fiscal years ended June 30, 2020, June 30, 2019, and June 30, 2018 was $13.69 , $27.96 , $46.62 and $32.67 , respectively.
The fair value of restricted stock units that vested during the transition period ended December 31, 2020, and the fiscal years ended June 30, 2020, June 30, 2019, and June 30, 2018 was $29.1 million, $32.4 million, $27.6 million and $20.4 million, respectively.
As of December 31, 2020, there was $54.3 million of total unrecognized share-based compensation expense related to RSUs that will be recognized over a weighted-average period of 2.7 years. We expect all unvested awards to vest and recognize forfeitures as they occur.
86
Share-based compensation expense recognized and included in the Consolidated Statements of Operations were as follows:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| Cost of molecular diagnostic testing | $ | $ | $ | $ | |||||||||||||||||||
| Cost of pharmaceutical and clinical services | |||||||||||||||||||||||
| Research and development expense | |||||||||||||||||||||||
| Selling, general, and administrative expense | |||||||||||||||||||||||
| Total share-based compensation expense | $ | $ | $ | $ | |||||||||||||||||||
The Company has unrecognized share-based compensation cost related to share-based compensation granted under its current plans. The estimated unrecognized share-based compensation cost and related weighted average recognition period, aggregate intrinsic value of options outstanding, aggregate intrinsic value of options that are fully vested and aggregate intrinsic value of RSUs vested and expected to vest is as follows:
| (in millions) | As of December 31, 2020 | ||||
| Unrecognized share-based compensation cost | $ | ||||
| Aggregate intrinsic value of options outstanding | |||||
| Aggregate intrinsic value of options fully vested | |||||
| Aggregate intrinsic value of RSUs outstanding | |||||
The total intrinsic value of options exercised was as follows:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| Total intrinsic value of options exercised | $ | $ | $ | $ | |||||||||||||||||||
Employee Stock Purchase Plan
On December 5, 2012, following stockholder approval, the Company adopted the 2012 Employee Stock Purchase Plan (the “2012 Purchase Plan”), under which 2.0 million shares of common stock have been authorized. Shares are issued under the 2012 Purchase Plan twice yearly at the end of each offering period. At December 31, 2020, a total of 1.8 million shares of common stock had been purchased under the 2012 Plan. Shares purchased under and compensation expense associated with the 2012 Plan for the years reported are as follows:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| Shares purchased under the plans | |||||||||||||||||||||||
| Plan compensation expense | $ | $ | $ | $ | |||||||||||||||||||
From June 1, 2017 through May 31, 2018 there was an amendment to the 2012 Purchase Plan implemented such that the plan was non-compensatory. As of December 31, 2020, there is $0.5 million unrecognized share-based compensation expense related to the 2012 Purchase Plan.
87
The fair value of shares issued under the Plan that was in effect for each period reported was calculated using the Black‑Scholes option-pricing model using the following weighted-average assumptions:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| 2020 | 2020 | 2019 | 2018 | ||||||||||||||||||||
| Risk-free interest rate | |||||||||||||||||||||||
| Expected dividend yield | |||||||||||||||||||||||
| Expected life (in years) | |||||||||||||||||||||||
| Expected volatility | |||||||||||||||||||||||
11. INCOME TAXES
Income tax benefit consists of the following:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| Current: | |||||||||||||||||||||||
| Federal | $ | ( | $ | $ | ( | $ | |||||||||||||||||
| State | ( | ( | |||||||||||||||||||||
| Foreign | |||||||||||||||||||||||
| Total current | ( | ( | |||||||||||||||||||||
| Deferred: | |||||||||||||||||||||||
| Federal | ( | ( | |||||||||||||||||||||
| State | ( | ( | |||||||||||||||||||||
| Foreign | ( | ( | ( | ||||||||||||||||||||
| Change in valuation allowance | ( | ||||||||||||||||||||||
| Total deferred | ( | ( | |||||||||||||||||||||
| Total income tax benefit | $ | ( | $ | ( | $ | ( | $ | ( | |||||||||||||||
Income (loss) before income taxes consists of the following:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| United States | $ | ( | $ | ( | $ | ( | $ | ||||||||||||||||
| Foreign | ( | ||||||||||||||||||||||
| Total | $ | ( | $ | ( | $ | $ | |||||||||||||||||
88
The differences between income taxes at the statutory federal income tax rate and income taxes reported in the consolidated statements of operations were as follows:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||||||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||||||||||||||||||||||||||
Federal income tax expense at the statutory rate | $ | ( | % | $ | ( | % | $ | % | $ | % | |||||||||||||||||||||||||||||||||||||
| State income taxes, net of federal benefit | ( | % | ( | % | % | % | |||||||||||||||||||||||||||||||||||||||||
Research and development credits, net of the federal tax on state credits | ( | % | ( | % | ( | ( | % | ( | ( | % | |||||||||||||||||||||||||||||||||||||
| Uncertain tax positions, net of federal benefit | ( | % | ( | % | % | % | |||||||||||||||||||||||||||||||||||||||||
Uncertain tax benefits statute expired, net of federal benefit | ( | % | ( | % | ( | ( | % | % | |||||||||||||||||||||||||||||||||||||||
Incentive stock option and employee stock purchase plan expense | ( | % | ( | % | ( | ( | % | ( | ( | % | |||||||||||||||||||||||||||||||||||||
| Foreign rate differential | ( | % | ( | % | % | ( | ( | % | |||||||||||||||||||||||||||||||||||||||
| Change in valuation allowance | ( | % | ( | % | ( | ( | % | % | |||||||||||||||||||||||||||||||||||||||
| Tax Cut and Jobs Act impact | % | % | % | ( | ( | % | |||||||||||||||||||||||||||||||||||||||||
| CARES Act | ( | % | — | — | % | — | — | % | — | — | % | ||||||||||||||||||||||||||||||||||||
Fair value adjustments related to acquisition contingent consideration | % | % | % | ( | ( | % | |||||||||||||||||||||||||||||||||||||||||
| Non-deductible contingent purchase price and transaction costs | ( | % | ( | % | % | % | |||||||||||||||||||||||||||||||||||||||||
| Non-deductible meals and entertainment | ( | % | ( | % | % | % | |||||||||||||||||||||||||||||||||||||||||
| Non-deductible officer compensation | ( | % | ( | % | % | % | |||||||||||||||||||||||||||||||||||||||||
| Asset impairment | % | ( | % | % | % | ||||||||||||||||||||||||||||||||||||||||||
| Expired tax attributes | % | ( | % | % | % | ||||||||||||||||||||||||||||||||||||||||||
| Non-deductible legal settlement | % | % | % | % | |||||||||||||||||||||||||||||||||||||||||||
| Foreign disregarded election | % | % | % | % | |||||||||||||||||||||||||||||||||||||||||||
| Changes in revenue recognition/method | % | % | ( | ( | % | % | |||||||||||||||||||||||||||||||||||||||||
| Other, net | % | ( | % | % | % | ||||||||||||||||||||||||||||||||||||||||||
| Total income tax benefit | $ | ( | % | $ | ( | % | $ | ( | - | % | $ | ( | - | % | |||||||||||||||||||||||||||||||||
89
The significant components of the Company’s deferred tax assets and liabilities were comprised of the following:
| December 31, | June 30, | ||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | ||||||||||||||
| Deferred tax assets: | |||||||||||||||||
| Net operating loss carryforwards | $ | $ | $ | ||||||||||||||
| Property, plant and equipment | |||||||||||||||||
| Accrued vacation | |||||||||||||||||
| AR allowance | |||||||||||||||||
| Stock compensation expense | |||||||||||||||||
| Research and development credits | |||||||||||||||||
| Lease right-of-use asset | |||||||||||||||||
| Uncertain state tax positions | |||||||||||||||||
| Other, net | |||||||||||||||||
| Total gross deferred tax assets | |||||||||||||||||
| Less valuation allowance | ( | ( | ( | ||||||||||||||
| Total deferred tax assets | |||||||||||||||||
| Deferred tax liabilities: | |||||||||||||||||
| Intangible assets | |||||||||||||||||
| Lease liability | |||||||||||||||||
| Property, plant and equipment | |||||||||||||||||
| Deferred revenue | |||||||||||||||||
| Total deferred tax liabilities | |||||||||||||||||
| Net deferred tax liability | $ | ( | $ | ( | $ | ( | |||||||||||
On March 27, 2020, the Coronavirus Aid, Relief and Economic Security Act (“CARES Act”) was enacted in response to COVID-19 pandemic. The CARES Act made various tax law changes, including among other things (i) increased the limitation under IRC Section 163(j) for 2019 and 2020 to permit additional expensing of interest (ii), enacted technical corrections so that qualified improvement property can be immediately expensed under IRC Section 168(k) and net operating losses arising in fiscal tax years beginning before January 1, 2018 and ending after December 31, 2017 can be carried back two years and carried forward twenty years without a taxable income limitation as opposed to carried forward indefinitely, and (iii) made modifications to the federal net operating loss rules including permitting federal net operating losses incurred in 2018, 2019, and 2020 to be carried back to the five preceding taxable years. As a result of the provision provided under the CARES Act, the Company was able to carry-back federal net operating losses to previous periods, resulting in a $20.7 million tax benefit in the transition period ended December 31, 2020.
On December 22, 2017, the Tax Cuts and Jobs Act (the “Tax Act”) was enacted. The Tax Act makes broad and complex changes to the U.S. tax code that are affecting our fiscal year ending June 30, 2018, and all following fiscal years. The Company’s final analysis of the impact of the Tax Cuts and Jobs Act resulted in a net income tax benefit during the fiscal year ended June 30, 2018 of approximately $32.0 million ($0.44 earnings per share increase). This amount primarily consisted of a net benefit for the corporate rate reduction due to the revaluing of net deferred tax liabilities as a result of the reduction in the federal corporate tax rates.
The Company determined that the provisions of the Tax Act affecting foreign earnings had no material effect or were not applicable to the Company’s fiscal years ended June 30, 2018, 2019, 2020, or to the transition period ended December 31, 2020. This was primarily due to the Company's election made during the fiscal year ended June 30, 2019 to treat its foreign subsidiaries as disregarded entities.
90
As a result of the economic impact of COVID-19, the Company has incurred a cumulative three-year loss. Pursuant to Accounting Standards Codification Topic 740, the negative evidence of a cumulative loss may be difficult to overcome. However, the Company will have significant future taxable income resulting from the reversal of taxable temporary differences. Primarily due to the availability of such expected future taxable income, the Company concluded that it is more likely than not that the benefits of the majority of its deferred income tax assets will be realized. However, for certain deferred tax assets a valuation allowance has been established, primarily due to limitations imposed by I.R.C. Section 382 and certain jurisdictional limitations. For the transition period ended December 31, 2020, the Company's valuation allowance increased by $0.3 million. For the fiscal years ended June 30, 2020, 2019 and 2018, the Company’s valuation allowance increased by $3.5 million, $1.1 million, and $0.6 million, respectively. The Company will continue to evaluate the impact that the COVID-19 pandemic may have on its results of operations and its ability to realize its deferred tax assets.
The Company acquired Counsyl, Inc. on July 31, 2018 (see Note 17). As part of the purchase accounting for the acquisition, a net deferred tax liability of approximately $67.6 million was recorded, consisting primarily of intangible assets for which the book basis exceeds the tax basis. A corresponding deferred tax asset of $60.7 million was recorded, consisting primarily of net operating loss and research credit carryforwards.
At December 31, 2020, the Company had the following net operating loss and research credit carryforwards (tax effected), with their respective expiration periods. Certain carryforwards are subject to the limitations of Section 382 and 383 of the Internal Revenue Code as indicated (in millions):
| Carryforwards | Amount | Subject to sections 382, 383 | Expires beginning in year | Through | ||||||||||||||||||||||
| Federal net operating loss | $ | |||||||||||||||||||||||||
| Utah net operating loss | Indefinite | |||||||||||||||||||||||||
| California net operating loss | ||||||||||||||||||||||||||
| Other state net operating loss | ||||||||||||||||||||||||||
| Foreign net operating losses (various jurisdictions) | Various | Various | ||||||||||||||||||||||||
| Federal research credit | ||||||||||||||||||||||||||
| Utah research credit | ||||||||||||||||||||||||||
| California research credit | Indefinite | |||||||||||||||||||||||||
| Texas research credit | ||||||||||||||||||||||||||
Consistent with the indefinite reversal criteria of ASC 740-30-25-17, the Company intends to continue to invest undistributed earnings of its foreign subsidiaries indefinitely. However, due to the cumulative losses that have been incurred to date in such foreign operations, the changes of the Tax Act and the aforementioned election to treat its foreign subsidiaries as disregarded entities, no deferred taxes related to the Company’s foreign operations have been recorded. For those foreign entities for which an election has been made to be treated as disregarded for U.S. tax purposes, the appropriate U.S. jurisdiction deferred tax assets and liabilities have been recorded.
91
The Company provides for uncertain tax positions when such tax positions do not meet the recognition thresholds or measurement criteria as set forth in ASC 740. As of December 31, 2020 and June 30, 2020, the Company had net unrecognized tax benefits of $30.5 million and $23.5 million, respectively. The Company’s gross unrecognized tax benefits as of the transition period ended December 31, 2020 and the fiscal years ended June 30, 2020, 2019 and 2018, and the changes in those balances are as follows:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| Unrecognized tax benefits at the beginning of period | $ | $ | $ | $ | |||||||||||||||||||
| Gross increases - current year tax positions | |||||||||||||||||||||||
| Gross increases - prior year tax positions | |||||||||||||||||||||||
| Gross increases - acquisitions | |||||||||||||||||||||||
| Gross decreases - prior year tax positions | ( | ( | ( | ||||||||||||||||||||
| Gross decreases - settlements | ( | ||||||||||||||||||||||
| Gross decreases - statute lapse | ( | ( | ( | ||||||||||||||||||||
| Unrecognized tax benefits at end of year | $ | $ | $ | $ | |||||||||||||||||||
| Interest and penalties in year-end balance | $ | $ | $ | $ | |||||||||||||||||||
Interest and penalties related to uncertain tax positions are included as a component of income tax expense and all other interest and penalties are included as a component of other income (expense).
The Company files U.S. federal, foreign and state income tax returns in jurisdictions with various statutes of limitations and is subject to examination for the open tax years in the U.S. federal and state jurisdictions of 2015 through 2020 and in the foreign jurisdictions of 2013 through 2020 . The Company is currently under audit by the State of New Jersey for the fiscal years June 30, 2013 through 2017 ; the city of New York for the fiscal years June 30, 2016 through 2018 ; Germany for the fiscal years June 30, 2013 through 2015 ; and Switzerland for the fiscal years June 30, 2015 through 2016 . Annual tax provisions include amounts considered necessary to pay assessments that may result from examination of prior year tax returns; however, the amount ultimately paid upon resolution of issues may differ materially from the amount accrued.
12. COMMITMENTS AND CONTINGENCIES
The Company is subject to various claims and legal proceedings covering matters that arise in the ordinary course of its business activities. As of December 31, 2020, management of the Company believes any liability that may ultimately result from the resolution of these matters will not have a material adverse effect on the Company’s consolidated financial position, operating results, or cash flows.
From time to time, the Company received recoupment requests from third-party payors for alleged overpayments. The Company disagrees with the contentions of the pending requests or has recorded an estimated reserve for the alleged overpayments.
13. LEASES
92
On July 1, 2019, the Company adopted ASU 2016-02 under the modified retrospective approach by initially applying ASU 2016-02 at the adoption date, rather than at the beginning of the earliest comparative period presented. Results for the fiscal year ended June 30, 2020 are presented under ASU 2016-02. Prior period amounts were not adjusted and continue to be reported under previous lease accounting guidance. As part of the adoption, the Company elected the package of practical expedients to avoid reassessing prior conclusions about lease identification, lease classification and initial direct costs. The Company also elected the practical expedient allowing the use of hindsight in determining the lease term and assessing impairment of right-of-use assets based on all facts and circumstances through the effective date of the new standard. The Company has elected the recognition exemption for short-term leases for all leases that qualify. Under this exemption, the Company will not recognize right-of-use assets or lease liabilities for those leases that qualify as a short-term lease (leases with lease terms of 12 months or less), which includes not recognizing right-of-use assets or lease liabilities for existing short-term leases in transition. The Company also has elected the practical expedient to avoid separating lease and non-lease components for any of its leases within its existing classes of assets.
The Company performed evaluations of its contracts and determined each of its identified leases are operating leases. For the transition period ended December 31, 2020, the Company incurred $9.9 million in lease costs which are included in operating expenses in the consolidated statement of operations in relation to these operating leases. Of such lease costs, $1.8 million was variable lease expense and $0.1 million was short-term lease expense, neither of which were included in the measurement of the Company's operating right of use assets and lease liabilities. The variable rent expense is comprised primarily of the Company's proportionate share of operating expenses, property taxes, and insurance and is classified as lease expense due to the Company's election to not separate lease and non-lease components. For the fiscal year ended June 30, 2020, the Company incurred $18.4 million in lease costs, of which $2.6 million was variable lease expense and $0.2 million was short-term lease expense. Prior to the adoption of the lease guidance in ASU 2016-02, the Company's total rent expense for the fiscal years ended June 30, 2019 and 2018 was $19.7 million and $15.5 million, respectively.
As of December 31, 2020, the maturities of the Company’s operating lease liabilities were as follows (in millions):
| Fiscal year ending: | |||||
| 2021 | $ | ||||
| 2022 | |||||
| 2023 | |||||
| 2024 | |||||
| 2025 | |||||
| Thereafter | |||||
| Total future lease payments | |||||
| Less: amounts representing interest | ( | ||||
| Present value of future lease payments | |||||
| Less: current maturities of operating lease liabilities | |||||
| Noncurrent operating lease liabilities | $ | ||||
As of December 31, 2020, the weighted average remaining lease term is 4.9 years and the weighted average discount rate used to determine the operating lease liability was 3.87 %.
As the implicit rate in the Company's leases is generally unknown, the Company uses its incremental borrowing rate based on the information available at the lease commencement date in determining the present value of future lease payments. When calculating the Company’s incremental borrowing rates, the Company gives consideration to its credit risk, term of the lease, total lease payments and adjust for the impacts of collateral, as necessary. The lease term used may reflect any option to extend or terminate the lease when it is reasonably certain the Company will exercise such options. Lease expenses for the Company's operating leases are recognized on a straight-line basis over the lease term.
14. EMPLOYEE DEFERRED SAVINGS PLAN
93
The Company’s recorded contributions to the plan are as follows:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| Deferred savings plan contributions | $ | $ | $ | $ | |||||||||||||||||||
15. SEGMENT AND RELATED INFORMATION
The Company’s business is aligned with how the chief operating decision maker ("CODM") reviews performance and makes decisions in managing the Company. The Company’s operating segments have been aggregated into two reportable segments: (i) diagnostics and (ii) other. The diagnostics segment primarily provides testing and collaborative development of testing that is designed to assess an individual’s risk for developing disease later in life, identify a patient’s likelihood of responding to drug therapy and guide a patient’s dosing to ensure optimal treatment, or assess a patient’s risk of disease progression and disease recurrence. The other segment provides testing products and services to the pharmaceutical, biotechnology and medical research industries, research and development, and clinical services for patients, and includes corporate services such as finance, human resources, legal and information technology. Segment information is consistent with how management reviews the business, makes investing and resource allocation decisions and assesses operating performance. The CODM evaluates segment performance based on operating income (loss).
| (in millions) | Diagnostics | Other | Total | ||||||||||||||
| Transition Period Ended December 31, 2020: | |||||||||||||||||
| Revenues | $ | $ | $ | ||||||||||||||
| Depreciation and amortization | |||||||||||||||||
| Segment operating loss | ( | ( | ( | ||||||||||||||
| Fiscal Year Ended June 30, 2020: | |||||||||||||||||
| Revenues | $ | $ | $ | ||||||||||||||
| Depreciation and amortization | |||||||||||||||||
| Segment operating loss | ( | ( | ( | ||||||||||||||
| Fiscal Year Ended June 30, 2019: | |||||||||||||||||
| Revenues | $ | $ | $ | ||||||||||||||
| Depreciation and amortization | |||||||||||||||||
| Segment operating income (loss) | ( | ||||||||||||||||
| Fiscal Year Ended June 30, 2018: | |||||||||||||||||
| Revenues | $ | $ | $ | ||||||||||||||
| Depreciation and amortization | |||||||||||||||||
| Segment operating income (loss) | ( | ||||||||||||||||
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| Total operating income (loss) for reportable segments | $ | ( | $ | ( | $ | $ | |||||||||||||||||
| Unallocated amounts: | |||||||||||||||||||||||
| Interest income | |||||||||||||||||||||||
| Interest expense | ( | ( | ( | ( | |||||||||||||||||||
| Other | ( | ( | |||||||||||||||||||||
| Income (loss) from operations before income taxes | ( | ( | |||||||||||||||||||||
| Income tax benefit | ( | ( | ( | ( | |||||||||||||||||||
| Net income (loss) | ( | ( | |||||||||||||||||||||
| Net loss attributable to non-controlling interest | ( | ( | ( | ||||||||||||||||||||
Net income (loss) attributable to Myriad Genetics, Inc stockholders | $ | ( | $ | ( | $ | $ | |||||||||||||||||
94
The following table sets forth a comparison of balance sheet assets by operating segment:
| December 31, | June 30, | ||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | ||||||||||||||
| Net equipment, leasehold improvements and property: | |||||||||||||||||
| Diagnostics | $ | $ | $ | ||||||||||||||
| Other | |||||||||||||||||
| Total | $ | $ | $ | ||||||||||||||
| Total assets less cash, cash equivalents, and marketable investment securities: | |||||||||||||||||
| Diagnostics | $ | $ | $ | ||||||||||||||
| Other | |||||||||||||||||
| Total | $ | $ | $ | ||||||||||||||
The following table reconciles assets by geographical region to total assets:
| December 31, | June 30, | ||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | ||||||||||||||
| Net equipment, leasehold improvements and property: | |||||||||||||||||
| United States | $ | $ | $ | ||||||||||||||
| Rest of world | |||||||||||||||||
| Total | $ | $ | $ | ||||||||||||||
| Total assets: | |||||||||||||||||
| United States | $ | $ | $ | ||||||||||||||
| Rest of world | |||||||||||||||||
| Total assets by segment and geographical region | $ | $ | $ | ||||||||||||||
| Cash, cash equivalents, and marketable investment securities (1) | |||||||||||||||||
| Total | $ | $ | $ | ||||||||||||||
(1)The Company manages cash, cash equivalents, and marketable investment securities at the consolidated level for all segments.
The following table reconciles revenue by geographical region to total revenue:
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| United States | $ | $ | $ | $ | |||||||||||||||||||
| Rest of world | |||||||||||||||||||||||
| Total revenue | $ | $ | $ | $ | |||||||||||||||||||
16. SALE OF SUBSIDIARY
95
17. BUSINESS ACQUISITIONS
Counsyl
On July 31, 2018, the Company completed the acquisition of Counsyl, Inc. (“Counsyl”), a leading provider of genetic testing and DNA analysis services, pursuant to the Agreement and Plan of Merger (the “Merger Agreement”), dated May 25, 2018. Pursuant to the terms of the Merger Agreement, Myriad Merger Sub, Inc., a newly created wholly-owned subsidiary of the Company, was merged with and into Counsyl, with Counsyl continuing as the surviving corporation and a wholly-owned subsidiary of Myriad. The Company believes the acquisition allows for further entry into the high-growth reproductive testing market, with the ability to become a leader in women’s health genetic testing.
The Company acquired Counsyl for total consideration of $405.9 million, consisting of $278.5 million in cash, financed in part by the Amendment No. 1 to the Facility (see Note 7) and 3.0 million shares of common stock issued, valued at $127.4 million. The shares were issued and valued as of July 31, 2018 at a per share market closing price of $42.53 .
Of the cash consideration, $5.0 million was deposited into an escrow account to fund any post-closing adjustments payable to Myriad based upon differences between the estimated working capital and the actual working capital of Counsyl at closing. The working capital was finalized during the fiscal year ended June 30, 2019 as described below.
Consideration transferred was allocated to the tangible and intangible assets acquired and liabilities assumed based on their fair values as of the acquisition date. Management estimated the fair value of tangible and intangible assets and liabilities in accordance with the applicable accounting guidance for business combinations and utilized the services of third-party valuation consultants. The significant assumptions used in the model to estimate the value of the intangible assets included projected cash flows, discount rates, net working capital and long-term growth rate. The initial allocation of the consideration transferred is based on a preliminary valuation and is subject to adjustments. Balances subject to adjustment primarily include the valuations of acquired assets (tangible and intangible), liabilities assumed, as well as tax-related matters. During the measurement period, the Company may record adjustments to the provisional amounts recognized. During the fiscal year ended June 30, 2019, $1.1 million of this escrow was returned to Myriad as a result of a working capital adjustment which reduced the total consideration and goodwill. There was also a reduction in the intangible assets of $2.9 million due to updated assumptions related to contributory asset charges associated with the related acquired asset, a $1.9 million decrease in the deferred tax liability, and a $0.7 million reduction to equipment due to updated valuations. The offset for the intangible asset, deferred tax liability and equipment changes was a $4.4 million increase in goodwill. The allocation of the consideration transferred was finalized within the measurement period.
The following table details the estimated fair value of total consideration transferred:
| (in millions) | Estimated Fair Value | ||||
| Current assets | $ | ||||
| Intangible assets | |||||
| Equipment | |||||
| Other assets | |||||
| Goodwill | |||||
| Current liabilities | ( | ||||
| Long term liabilities | ( | ||||
| Deferred tax liability | ( | ||||
| Total fair value purchase price | $ | ||||
| Less: Cash acquired | ( | ||||
| Total consideration transferred | $ | ||||
Identifiable Intangible Assets
The Company acquired intangible assets that consisted of developed screening processes, which had an estimated fair value of $290.0 million. The fair values of these developed screening processes and related useful lives were determined using a probability-weighted income approach that discounts expected future cash flows to present value. The estimated net cash flows were discounted using a discount rate of 12.5 %, which is based on the estimated internal rate of return for the acquisition and represents the rate that market participants might use to value the intangible assets. The Company will amortize the intangible assets on a straight-line basis over their estimated useful lives of 12 years.
96
Goodwill
The goodwill represents the excess of consideration transferred over the fair value of assets acquired and liabilities assumed and is attributable to the benefits expected from combining the Company’s expertise with Counsyl’s technology, customer insights, and ability to effectively integrate genetic screening into clinical practice with OBGYNs. Changes in goodwill since the initial purchase are shown below:
| (in millions) | Carrying amount | ||||
| Balance September 30, 2018 | $ | ||||
| Fair value adjustment to equipment | |||||
| Intangible adjustment | |||||
| Working capital adjustment | ( | ||||
| Change in deferred tax liability | |||||
| Ending balance December 31, 2020 | $ | ||||
This goodwill is not deductible for income tax purposes.
Pro Forma Information (Unaudited)
The unaudited pro-forma results presented below include the effects of the Counsyl acquisition as if it had been consummated as of July 1, 2017, with adjustments to give effect to pro forma events that are directly attributable to the acquisition, which includes adjustments related to the amortization of acquired intangible assets, interest income and expense, and depreciation.
The unaudited pro forma results do not reflect any operating efficiency or potential cost savings that may result from the consolidation of Counsyl with the Company. Accordingly, these unaudited pro forma results are presented for informational purposes only and are not necessarily indicative of what the actual results of operation of the combined company would have been if the acquisition had occurred at the beginning of the period presented, nor are they indicative of future results of operations and are not necessarily indicative of results that might have been achieved had the acquisition been consummated as of July 1, 2017.
| Years Ended June 30, | |||||||||||
| (in millions) | 2019 | 2018 | |||||||||
| Revenue | $ | $ | |||||||||
| Income from operations | |||||||||||
| Net income | |||||||||||
| Net income per share, basic | $ | $ | |||||||||
| Net income per share, diluted | $ | $ | |||||||||
To complete the purchase transaction, the Company incurred approximately $6.8 million of acquisition costs, which are recorded as selling, general and administrative expenses in the period incurred. For the fiscal year ended June 30, 2019, Counsyl contributed revenue of approximately $104.9 million. For the fiscal year ended June 30, 2019, operating expenses related to Counsyl were approximately $67.6 million.
97
18. SUBSEQUENT EVENT
On February 22, 2021, the Company entered into Amendment No. 3 to the Amended Facility discussed in Note 7, which modified the Amended Facility as follows:
•Extends the Modification Period established pursuant to Amendment No. 2 for an additional year, through June 30, 2022, and revises certain negative covenants in connection therewith.
•Restricts the Company from borrowing under the revolving credit facility if unrestricted cash and cash equivalents exceed $150.0 million, unless such borrowings are in connection with acquisitions.
◦Reduces the revolving commitments to $300.0 million, with a further reduction to $250.0 million by September 30, 2021. Provides for further reductions to the revolving commitments, or mandatory prepayments of outstanding revolving loans, in the event of certain asset sales.
•Waives compliance with the leverage ratio and the interest coverage ratio covenants through the quarter ended March 31, 2022 and also lowers the minimum liquidity covenant applicable through such quarter.
◦Removes the minimum EBITDA covenant added pursuant to Amendment No. 2.
◦Permits the Company to change its fiscal year end to December 31.
98
19. SUPPLEMENTAL CASH FLOW INFORMATION
| Transition Period Ended December 31, | Years Ended June 30, | ||||||||||||||||||||||
| (in millions) | 2020 | 2020 | 2019 | 2018 | |||||||||||||||||||
| Cash paid for income taxes | $ | $ | $ | $ | |||||||||||||||||||
| Cash paid for interest | |||||||||||||||||||||||
| Non-cash investing and financing activities: | |||||||||||||||||||||||
Fair value adjustment on marketable investment securities recorded to stockholders' equity | ( | ( | |||||||||||||||||||||
| Establishment of operation lease right-of-use assets and lease liabilities | |||||||||||||||||||||||
| Operating lease right-of-use assets | $ | — | $ | $ | — | $ | — | ||||||||||||||||
| Operating lease liabilities | — | ( | — | — | |||||||||||||||||||
| Accrued liabilities and other long-term liabilities | — | — | — | ||||||||||||||||||||
99
20. SUPPLEMENTARY QUARTERLY FINANCIAL DATA (UNAUDITED)
| (in millions) | Quarters Ended | ||||||||||
| Consolidated Statement of Operations Data: | December 31, 2020 | September 30, 2020 | |||||||||
| Molecular diagnostic testing | $ | $ | |||||||||
| Pharmaceutical and clinical services | |||||||||||
| Total revenue | |||||||||||
| Costs and expenses: | |||||||||||
| Cost of molecular diagnostic testing | |||||||||||
| Cost of pharmaceutical and clinical services | |||||||||||
| Research and development expense | |||||||||||
| Change in the fair value of contingent consideration | ( | ||||||||||
| Selling, general and administrative expense | |||||||||||
| Total costs and expenses | |||||||||||
| Operating loss | ( | ( | |||||||||
| Other income (expense): | |||||||||||
| Interest income | |||||||||||
| Interest expense | ( | ( | |||||||||
| Other | ( | ||||||||||
| Total other expense | ( | ( | |||||||||
| Loss before income taxes | ( | ( | |||||||||
| Income tax benefit | ( | ( | |||||||||
| Net loss | ( | ( | |||||||||
| Net loss attributable to non-controlling interest | |||||||||||
| Net loss attributable to Myriad Genetics Inc. stockholders | $ | ( | $ | ( | |||||||
| Loss per share: | |||||||||||
| Basic | $ | ( | $ | ( | |||||||
| Diluted | $ | ( | $ | ( | |||||||
| Weighted average shares outstanding: | |||||||||||
| Basic | |||||||||||
| Diluted | |||||||||||
100
| (in millions) | Quarters Ended | ||||||||||||||||||||||
| Consolidated Statement of Operations Data: | June 30, 2020 | March 31, 2020 | December 31, 2019 (a) | September 30, 2019 | |||||||||||||||||||
| Molecular diagnostic testing | $ | $ | $ | $ | |||||||||||||||||||
| Pharmaceutical and clinical services | |||||||||||||||||||||||
| Total revenue | |||||||||||||||||||||||
| Costs and expenses: | |||||||||||||||||||||||
| Cost of molecular diagnostic testing | |||||||||||||||||||||||
| Cost of pharmaceutical and clinical services | |||||||||||||||||||||||
| Research and development expense | |||||||||||||||||||||||
| Change in the fair value of contingent consideration | ( | ( | |||||||||||||||||||||
| Selling, general and administrative expense | |||||||||||||||||||||||
| Goodwill and intangible asset impairment charges | |||||||||||||||||||||||
| Total costs and expenses | |||||||||||||||||||||||
| Operating loss | ( | ( | ( | ( | |||||||||||||||||||
| Other income (expense): | |||||||||||||||||||||||
| Interest income | |||||||||||||||||||||||
| Interest expense | ( | ( | ( | ( | |||||||||||||||||||
| Other | ( | ||||||||||||||||||||||
| Total other income (expense) | ( | ( | |||||||||||||||||||||
| Loss before income taxes | ( | ( | ( | ( | |||||||||||||||||||
| Income tax benefit | ( | ( | ( | ( | |||||||||||||||||||
| Net loss | ( | ( | ( | ( | |||||||||||||||||||
| Net loss attributable to non-controlling interest | ( | ||||||||||||||||||||||
Net loss attributable to Myriad Genetics Inc. stockholders | $ | ( | $ | ( | $ | ( | $ | ( | |||||||||||||||
| Loss per share: | |||||||||||||||||||||||
| Basic | $ | ( | $ | ( | $ | ( | $ | ( | |||||||||||||||
| Diluted | $ | ( | $ | ( | $ | ( | $ | ( | |||||||||||||||
| Weighted average shares outstanding: | |||||||||||||||||||||||
| Basic | |||||||||||||||||||||||
| Diluted | |||||||||||||||||||||||
(a) An immaterial prior period goodwill impairment charge of $1.3
101
| (in millions) | Quarters Ended | ||||||||||||||||||||||
| Consolidated Statement of Operations Data: | June 30, 2019 | March 31, 2019 | December 31, 2018 | September 30, 2018 | |||||||||||||||||||
| Molecular diagnostic testing | $ | $ | $ | $ | |||||||||||||||||||
| Pharmaceutical and clinical services | |||||||||||||||||||||||
| Total revenue | |||||||||||||||||||||||
| Costs and expenses: | |||||||||||||||||||||||
| Cost of molecular diagnostic testing | |||||||||||||||||||||||
| Cost of pharmaceutical and clinical services | |||||||||||||||||||||||
| Research and development expense | |||||||||||||||||||||||
| Change in contingent consideration | ( | ||||||||||||||||||||||
| Selling, general and administrative expense | |||||||||||||||||||||||
| Total costs and expenses | |||||||||||||||||||||||
| Operating income (loss) | ( | ||||||||||||||||||||||
| Other income (expense): | |||||||||||||||||||||||
| Interest income | |||||||||||||||||||||||
| Interest expense | ( | ( | ( | ( | |||||||||||||||||||
| Other | ( | ||||||||||||||||||||||
| Total other expense | ( | ( | ( | ( | |||||||||||||||||||
| Income (loss) before income taxes | ( | ||||||||||||||||||||||
| Income tax provision (benefit) | ( | ( | |||||||||||||||||||||
| Net income (loss) | ( | ( | |||||||||||||||||||||
| Net loss attributable to non-controlling interest | ( | ( | |||||||||||||||||||||
Net income (loss) attributable to Myriad Genetics, Inc. stockholders | $ | ( | $ | $ | $ | ( | |||||||||||||||||
| Earnings (loss) per share: | |||||||||||||||||||||||
| Basic | $ | ( | $ | $ | $ | ( | |||||||||||||||||
| Diluted | $ | ( | $ | $ | $ | ( | |||||||||||||||||
| Weighted average shares outstanding: | |||||||||||||||||||||||
| Basic | |||||||||||||||||||||||
| Diluted | |||||||||||||||||||||||
102
21. TRANSITION PERIOD COMPARATIVE DATA (UNAUDITED)
| (in millions) | Six-month Period Ended | ||||
| Consolidated Statement of Operations Data: | December 31, 2019 | ||||
| Molecular diagnostic testing | $ | ||||
| Pharmaceutical and clinical services | |||||
| Total revenue | |||||
| Costs and expenses: | |||||
| Cost of molecular diagnostic testing | |||||
| Cost of pharmaceutical and clinical services | |||||
| Research and development expense | |||||
| Change in the fair value of contingent consideration | |||||
| Selling, general and administrative expense | |||||
| Goodwill and intangible asset impairment charges | |||||
| Total costs and expenses | |||||
| Operating loss | ( | ||||
| Other income (expense): | |||||
| Interest income | |||||
| Interest expense | ( | ||||
| Other | ( | ||||
| Total other expense | ( | ||||
| Loss before income taxes | ( | ||||
| Income tax benefit | ( | ||||
| Net loss | ( | ||||
| Net loss attributable to non-controlling interest | |||||
| Net loss attributable to Myriad Genetics Inc. stockholders | $ | ( | |||
| Loss per share: | |||||
| Basic | $ | ( | |||
| Diluted | $ | ( | |||
| Weighted average shares outstanding: | |||||
| Basic | |||||
| Diluted | |||||
103
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
Item 9A. CONTROLS AND PROCEDURES
1. Disclosure Controls and Procedures
We maintain disclosure controls and procedures (Disclosure Controls) within the meaning of Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended, or the Exchange Act. Our Disclosure Controls are designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act, such as this Transition Report on Form 10-K, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms. Our Disclosure Controls are also designed to ensure that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating our Disclosure Controls, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management necessarily applied its judgment in evaluating and implementing possible controls and procedures.
As of the end of the period covered by this Transition Report on Form 10-K, we evaluated the effectiveness of the design and operation of our Disclosure Controls, which was done under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer. Based on the evaluation of our Disclosure Controls, our Chief Executive Officer and Chief Financial Officer has concluded that, as of December 31, 2020, our Disclosure Controls were not effective due to a material weakness in the Company's internal control over financial reporting as disclosed below.
2. Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rules 13a‑15(f) and 15d‑15(f) under the Exchange Act. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States.
In connection with the preparation of our consolidated financial statements as of and for the transition period ended December 31, 2020, we identified a material weakness in controls over the accounting for intercompany transactions, foreign currency exchanges and foreign currency translation related to our international subsidiaries. Specifically, as part of our financial statement close process, certain of our control activities were not sufficiently designed or operating effectively to ensure all of our policies were in compliance with generally accepted accounting principles, consistent in their application, retained in appropriate documentation and communicated to relevant parties. As a result of the material weakness, we recorded certain immaterial corrections to intercompany accounts, as well as foreign currency exchange and translation gains and losses, in our consolidated financial statements for the transition period ended December 31, 2020.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies may deteriorate.
The effectiveness of our internal control over financial reporting as of December 31, 2020, has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report included elsewhere herein.
104
3. Plans to Remediate Material Weakness
We have begun the process of, and we are focused on, designing and implementing effective internal controls measures to improve our internal control over financial reporting and remediate the material weakness identified above. Our efforts include the following actions:
•We are designing and implementing application controls in our financial systems to improve the reliability of our process and reporting.
•We are designing and implementing additional review procedures within our accounting and finance department to provide more robust and comprehensive internal controls over financial reporting that address the risks of material misstatement related to the accounting for intercompany transactions, foreign currency exchanges and foreign currency translation within our business processes.
While these actions and planned actions are subject to ongoing management evaluation and will require validation and testing of the design and operating effectiveness of internal controls over a sustained period of financial reporting cycles, we are committed to the continuous improvement of our internal control over financial reporting and will continue to diligently review our internal control over financial reporting for that purpose.
4. Change in Internal Control over Financial Reporting
The Company is in the midst of a multi-year transformation project to achieve better analytics and process efficiencies through the use of Oracle Fusion Cloud Services System. During the transition period ended December 31, 2020, the Company completed the implementation of certain modules used in the financial statement close process and management reporting. Additional phases will continue to be implemented over the coming year. Emphasis will continue to be placed on the maintenance of effective internal controls and assessment of the design and operating effectiveness of key control activities throughout development and deployment of each phase.
Other than as described above, there were no changes in our internal control over financial reporting that occurred during the quarter or transition period ended December 31, 2020 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
5. Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of Myriad Genetics, Inc.
Opinion on Internal Control over Financial Reporting
We have audited Myriad Genetics, Inc. and subsidiaries’ internal control over financial reporting as of December 31, 2020, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). In our opinion, because of the effect of the material weakness described below on the achievement of the objectives of the control criteria, Myriad Genetics, Inc. and subsidiaries (the Company) has not maintained effective internal control over financial reporting as of December 31, 2020, based on the COSO criteria.
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The following material weakness has been identified and included in management’s assessment. Management identified a material weakness in controls over the accounting for intercompany transactions, foreign currency exchanges and foreign currency translation related to its international subsidiaries.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of Myriad Genetics, Inc. and subsidiaries as of December 31, 2020, June 30, 2020 and 2019, and the related consolidated statements of operations, comprehensive income, stockholders’ equity and cash flows for the transition period ended December 31, 2020 and each of the three years in the period ended June 30, 2020, and the related notes and our report dated March 16, 2021 expressed an unqualified opinion thereon.
105
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ Ernst & Young LLP
Salt Lake City, UT
March 16, 2021
Item 9B. OTHER INFORMATION
None.
106
PART III
Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Management and Corporate Governance,” “Delinquent Section 16(a) Reports” and “Corporate Code of Conduct and Ethics” in our Proxy Statement for the 2021 Annual Meeting of Stockholders to be held on June 3, 2021.
Item 11. EXECUTIVE COMPENSATION
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Executive Compensation,” “Management and Corporate Governance – Committees of the Board of Directors and Meetings – Compensation Committee Interlocks and Insider Participation,” “Compensation Committee Report” and “Management and Corporate Governance – Board’s Role in the Oversight of Risk Management” in our Proxy Statement for the 2021 Annual Meeting of Stockholders to be held on June 3, 2021.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Executive Compensation - Equity Compensation Plan Information” in our Proxy Statement for the 2021 Annual Meeting of Stockholders to be held on June 3, 2021.
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The response to this item is incorporated by reference from the discussion responsive thereto under the caption “Certain Relationships and Related Person Transactions” and “Management and Corporate Governance – Director Independence” in our Proxy Statement for the 2021 Annual Meeting of Stockholders to be held on June 3, 2021.
Item 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The response to this item is incorporated by reference from the discussion responsive thereto in the proposal entitled “Selection of Independent Registered Public Accounting Firm” in our Proxy Statement for the 2021 Annual Meeting of the Stockholders to be held on June 3, 2021.
107
PART IV
Item 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a)The following documents are included as part of this Transition Report on Form 10-K.
1. Financial Statements
See “Index to Consolidated Financial Statements” at Item 8 to this Transition Report on Form 10-K.
2. Financial Statement Schedules
Financial statement schedules have not been included because they are not applicable, or the information is included in financial statements or notes thereto.
3. Exhibits
| Exhibit Number | Exhibit Description | Filed with this Report | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Registration Number | |||||||||||||||||||||||||||
| 3.1 | 10-K (Exhibit 3.1) | 8/15/2011 | 000-26642 | |||||||||||||||||||||||||||||
| 3.2 | 8-K (Exhibit 3.1) | 10/15/2020 | 000-26642 | |||||||||||||||||||||||||||||
| 4.1 | 10-K (Exhibit 4.1) | 8/15/2011 | 000-26642 | |||||||||||||||||||||||||||||
| 4.2 | X | |||||||||||||||||||||||||||||||
| Lease Agreements | ||||||||||||||||||||||||||||||||
| 10.1 | .1 | 10-Q (Exhibit 10.2) | 11/8/1996 | 000-26642 | ||||||||||||||||||||||||||||
| .2 | 10-Q (Exhibit 10.1) | 5/4/2016 | 000-26642 | |||||||||||||||||||||||||||||
| 10.2 | .1 | 10-K (Exhibit 10.44) | 9/24/1998 | 000-26642 | ||||||||||||||||||||||||||||
| .2 | 10-Q (Exhibit 10.2) | 5/4/2016 | 000-26642 | |||||||||||||||||||||||||||||
| 10.3 | .1 | 10-Q (Exhibit 10.1) | 5/15/2001 | 000-26642 | ||||||||||||||||||||||||||||
| .2 | 10-Q (Exhibit 10.3) | 5/4/2016 | 000-26642 | |||||||||||||||||||||||||||||
| 10.4 | .1 | 8-K (Exhibit 99.1) | 7/5/2005 | 000-26642 | ||||||||||||||||||||||||||||
| .2 | 8-K (Exhibit 99.2) | 7/5/2005 | 000-26642 | |||||||||||||||||||||||||||||
108
| Exhibit Number | Exhibit Description | Filed with this Report | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Registration Number | |||||||||||||||||||||||||||
| .3 | 10-Q (Exhibit 10.4) | 5/4/2016 | 000-26642 | |||||||||||||||||||||||||||||
| 10.5 | .1 | 10-K (Exhibit 10.32) | 8/28/2008 | 000-26642 | ||||||||||||||||||||||||||||
| .2 | 10-Q (Exhibit 10.4) | 5/5/2010 | 000-26642 | |||||||||||||||||||||||||||||
| 10.6 | 10-K(Exhibit 10.6) | 8/13/2019 | 000-26642 | |||||||||||||||||||||||||||||
| Agreements with Executive Officers and Directors | ||||||||||||||||||||||||||||||||
| 10.7 | 10-Q (Exhibit 10.1) | 2/7/2020 | 000-26642 | |||||||||||||||||||||||||||||
| 10.8 | X | |||||||||||||||||||||||||||||||
| 10.9 | 10-K (Exhibit 10.34) | 8/25/2009 | 000-26642 | |||||||||||||||||||||||||||||
| 10.10 | 8-K (Exhibit 10.1) | 10/15/2020 | 000-26642 | |||||||||||||||||||||||||||||
| 10.11 | 10-Q (Exhibit 10.1) | 11/9/2020 | 000-26642 | |||||||||||||||||||||||||||||
| 10.12 | 10-Q (Exhibit 10.2) | 11/9/2020 | 000-26642 | |||||||||||||||||||||||||||||
| 10.13 | 10-Q (Exhibit 10.3) | 11/9/2020 | 000-26642 | |||||||||||||||||||||||||||||
| 10.14 | 10-Q (Exhibit 10.4) | 11/9/2020 | 000-26642 | |||||||||||||||||||||||||||||
| 10.15 | 10-Q (Exhibit 10.5) | 11/9/2020 | 000-26642 | |||||||||||||||||||||||||||||
| 10.16 | 10-Q (Exhibit 10.6) | 11/9/2020 | 000-26642 | |||||||||||||||||||||||||||||
| Equity Compensation Plans | ||||||||||||||||||||||||||||||||
| 10.17 | 8-K (Exhibit 10.1) | 12/2/2016 | 000-26642 | |||||||||||||||||||||||||||||
109
| Exhibit Number | Exhibit Description | Filed with this Report | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Registration Number | |||||||||||||||||||||||||||
| 10.18 | 10-Q (Exhibit 10.3) | 2/1/2011 | 000-26642 | |||||||||||||||||||||||||||||
| 10.19 | 10-Q (Exhibit 10.4) | 2/1/2011 | 000-26642 | |||||||||||||||||||||||||||||
| 10.20 | 10-Q (Exhibit 10.1) | 11/5/2014 | 000-26642 | |||||||||||||||||||||||||||||
| 10.21 | 8-K (Exhibit 10.1) | 12/7/2020 | 000-26642 | |||||||||||||||||||||||||||||
| 10.22 | 10-K (Exhibit 10.11) | 8/13/2020 | 000-26642 | |||||||||||||||||||||||||||||
| 10.23 | 8-K (Exhibit 10.2) | 12/7/2012 | 000-26642 | |||||||||||||||||||||||||||||
| 10.24 | 8-K (Exhibit 10.2) | 12/1/2017 | 000-26642 | |||||||||||||||||||||||||||||
| Credit Agreement | ||||||||||||||||||||||||||||||||
| 10.25 | 8-K (Exhibit 10.1) | 2/23/2021 | 000-26642 | |||||||||||||||||||||||||||||
| Merger Agreements | ||||||||||||||||||||||||||||||||
| 10.26 | 10-Q (Exhibit 10.1) | 11/2/2016 | 000-26642 | |||||||||||||||||||||||||||||
| 10.27 | 10-K (Exhibit 10.18) | 8/24/2018 | 000-26642 | |||||||||||||||||||||||||||||
| Other | ||||||||||||||||||||||||||||||||
| 21.1 | 10-K (Exhibit 21.1) | 8/13/2020 | 000-26642 | |||||||||||||||||||||||||||||
| 23.1 | X | |||||||||||||||||||||||||||||||
| 31.1 | X | |||||||||||||||||||||||||||||||
| 31.2 | X | |||||||||||||||||||||||||||||||
110
| Exhibit Number | Exhibit Description | Filed with this Report | Incorporated by Reference herein from Form or Schedule | Filing Date | SEC File/ Registration Number | |||||||||||||||||||||||||||
| 32 | X | |||||||||||||||||||||||||||||||
| 101 | The following materials from Myriad Genetics, Inc.’s Transition Report on Form 10-K for the transition period ended December 31, 2020, formatted in XBRL (Xtensible Business Reporting Language): (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Comprehensive Income, (iii) Consolidated Statements of Stockholders’ Equity, (iv) Consolidated Statements of Cash Flows, and (v) Notes to Consolidated Financial Statements. Inline XBRL Instance Document – Instance Document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. | X | ||||||||||||||||||||||||||||||
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document) | X | ||||||||||||||||||||||||||||||
(+) Management contract or compensatory plan arrangement.
(@) The agreements with all executives are identical except for the executive who is a party to the agreement and the date of execution, which are listed at the end of the exhibit.
Item 16. FORM 10-K SUMMARY
None.
111
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on March 16, 2021.
| MYRIAD GENETICS, INC. | ||||||||
| By: | /s/ Paul J. Diaz | |||||||
| Paul J. Diaz | ||||||||
| President and Chief Executive Officer | ||||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities indicated below and on the dates indicated.
| Signatures | Title | Date | |||||||||||||||
| President, Chief Executive Officer and Director (Principal Executive Officer) | |||||||||||||||||
| By: | /s/ Paul J. Diaz | March 16, 2021 | |||||||||||||||
| Paul J. Diaz | |||||||||||||||||
| Chief Financial Officer (Principal Financial and Accounting Officer) | |||||||||||||||||
| By: | /s/ R. Bryan Riggsbee | March 16, 2021 | |||||||||||||||
| R. Bryan Riggsbee | |||||||||||||||||
| By: | /s/ S. Louise Phanstiel | Chair of the Board | March 16, 2021 | ||||||||||||||
| S. Louise Phanstiel | |||||||||||||||||
| By: | /s/ Heiner Dreismann | Director | March 16, 2021 | ||||||||||||||
| Heiner Dreismann, Ph.D. | |||||||||||||||||
| By: | /s/ Rashmi Kumar | Director | March 16, 2021 | ||||||||||||||
| Rashmi Kumar | |||||||||||||||||
| By: | /s/ Dennis Langer | Director | March 16, 2021 | ||||||||||||||
| Dennis Langer, M.D., J.D. | |||||||||||||||||
| By: | /s/ Lee N. Newcomer | Director | March 16, 2021 | ||||||||||||||
| Lee N. Newcomer, M.D. | |||||||||||||||||
| By: | /s/ Colleen F. Reitan | Director | March 16, 2021 | ||||||||||||||
| Colleen F. Reitan | |||||||||||||||||
| By: | /s/ Daniel M. Skovronsky | Director | March 16, 2021 | ||||||||||||||
| Daniel M. Skovronsky, M.D., Ph.D. | |||||||||||||||||
| By: | /s/ Daniel K. Spiegelman | Director | March 16, 2021 | ||||||||||||||
| Daniel K. Spiegelman | |||||||||||||||||
112
DESCRIPTION OF THE REGISTRANT’S SECURITIES REGISTERED PURSUANT TO SECTION 12 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED
Myriad Genetics, Inc. (the “Company” or “we”) has one class of securities registered under Section 12 of the Securities Exchange Act of 1934, as amended: our common stock, par value $0.01 per share.
DESCRIPTION OF COMMON STOCK
The following description of our common stock is a summary and does not purport to be complete. You should refer to our Restated Certificate of Incorporation, as amended (“restated certificate of incorporation”) and our Restated By-laws (“restated bylaws”), both of which are incorporated by reference as exhibits to the Company’s Transition Report on Form 10‑K of which this exhibit is a part. The summary below is also qualified by provisions of the Delaware General Corporation Law.
Authorized Capital Stock
Under our restated certificate of incorporation our authorized capital stock consists of 150,000,000 shares of common stock, $0.01 par value per share, and 5,000,000 shares of preferred stock, $0.01 par value per share, of which 600,000 shares have been designated Series A Junior Participating Preferred Stock. We designated the Series A Junior Participating Preferred Stock in connection with a stockholders’ rights plan that has expired. Accordingly, we have no present intention to issue any shares of Series A Junior Participating Preferred Stock.
General
Holders of our common stock are entitled to one vote for each share of common stock held of record for the election of directors and on all matters submitted to a vote of stockholders. Holders of our common stock are entitled to receive dividends ratably, if any, as may be declared by our board of directors out of legally available funds, subject to any preferential dividend rights of any preferred stock then outstanding. Upon our dissolution, liquidation or winding up, holders of our common stock are entitled to share ratably in our net assets legally available after the payment of all of our debts and other liabilities, subject to the preferential rights of any preferred stock then outstanding. Holders of our common stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of our common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we have designated and may issue in the future or that we may designate and issue in the future. Except as described under “Certain Provisions of Delaware Law and of the Company’s Certificate of Incorporation and Bylaws” below, a majority vote of the holders of our common stock is generally required to take action under our restated certificate of incorporation and restated bylaws.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is American Stock Transfer & Trust Company, LLC, with offices at 6201 15th Avenue, Brooklyn, New York 11219.
Stock Exchange Listing
Our common stock is listed for quotation on The Nasdaq Global Select Market under the symbol “MYGN.”
CERTAIN PROVISIONS OF DELAWARE LAW AND OF THE COMPANY’S CERTIFICATE OF INCORPORATION AND BYLAWS
Anti-Takeover Provisions
The provisions of Delaware law and of our restated certificate of incorporation and restated bylaws discussed below could discourage or make it more difficult to accomplish a proxy contest or other change in our management or the acquisition of control by a holder of a substantial amount of our voting stock. It is possible that these provisions could make it more difficult to accomplish, or could deter, transactions that stockholders may otherwise consider to be in their best interests or the best interests of the Company.
Delaware Law
We are subject to Section 203 of the Delaware General Corporation Law. Subject to certain exceptions, Section 203 prevents a publicly held Delaware corporation from engaging in a “business combination” with any “interested stockholder” for a three-year period following the time that such stockholder becomes an interested stockholder, unless the interested stockholder attained such status with the approval of our board of directors or unless the business combination is approved in a prescribed manner. A “business combination” includes, among other things, a merger or consolidation involving us and the “interested stockholder” and the sale of more than 10% of our assets. In general, an “interested stockholder” is any entity or person beneficially owning 15% or more of our outstanding voting stock and any entity or person affiliated with or controlling or controlled by such entity or person.
Restated Certificate of Incorporation and Restated Bylaws
Our board of directors is divided into three classes. Each year our stockholders elect the members of one of the three classes to a term of office to expire at the third succeeding annual meeting of stockholders after their election. All directors elected to our classified board of directors serve until the election and qualification of their respective successors or their earlier resignation or removal. Only the board of directors is authorized to create new directorships and to fill such positions so created and is permitted to specify the class to which any such new position is assigned. The person filling such position would serve for the term applicable to that class. Only the board of directors (or its remaining members, even if less than a quorum) is empowered to fill vacancies on the board of directors occurring for any reason for the remainder of the term of the class of directors in which the vacancy occurred. Members of the board of directors may only be removed for cause. These provisions are likely to increase the time required for stockholders to change the composition of the board of directors. For example, in general, at least two annual meetings would be necessary for stockholders to effect a change in a majority of the members of the board of directors.
Our restated bylaws provide that, for nominations to the board of directors or for other business to be properly brought by a stockholder before a meeting of stockholders, the stockholder must first have given timely notice of the proposal in writing to our Secretary. For an annual meeting, a stockholder’s notice generally must be delivered not less than 60 days nor more than 90 days prior to the anniversary of the previous year’s annual meeting. For a special meeting, the notice must generally be delivered by not less than 60 days nor more than 90 days prior to the special meeting or ten days following the day on which public announcement of the meeting is first made. Detailed requirements as to the form of the notice and information required in the notice are specified in our restated bylaws. If it is determined that business was not properly brought before a meeting in accordance with the provisions of our restated bylaws, such business will not be conducted at the meeting.
Special meetings of the stockholders may be called only by the chair of our board of directors, the chief executive officer or president with the approval of the executive committee of the board of directors, or by the entire board of directors pursuant to a resolution adopted by a majority of the total number of authorized directors, whether or not there exists any vacancy in previously authorized directorships.
Our restated certificate of incorporation does not permit our stockholders to act by written consent. As a result, any action to be effected by our stockholders must be effected at a duly called annual or special meeting of the stockholders.
The Delaware General Corporation Law provides generally that the affirmative vote of a majority of the shares entitled to vote on any matter is required to amend a corporation’s certificate of incorporation or bylaws, unless the corporation’s certificate of incorporation or bylaws, as the case may be, requires a greater percentage. Our restated certificate of incorporation requires the affirmative vote of the holders of at least 70% of our outstanding voting stock to amend or repeal any of the provisions discussed above in this section entitled “Anti‑Takeover Provisions — Restated Certificate of Incorporation and Restated Bylaws.” This 70% stockholder vote would be in addition to any separate class vote that might in the future be required pursuant to the terms of any preferred stock that might then be outstanding. A 70% vote will also be required for any amendment to, or repeal of, our restated bylaws by the stockholders. Our restated bylaws may be amended or repealed by a simple majority vote of the board of directors.
Our board of directors is authorized, without action by our stockholders, to designate and issue shares of our preferred stock in one or more series and to designate the rights, preferences and privileges of the shares of each series and any of its qualifications, limitations or restrictions. The existence of authorized but unissued shares of preferred stock enables our board of directors to render more difficult or to discourage an attempt to obtain control of the Company by means of a merger, tender offer, proxy contest or otherwise.
MYRIAD GENETICS, INC.
NON-EMPLOYEE DIRECTOR COMPENSATION POLICY
(Effective: Fiscal Year 2021)
The following is a description of the standard compensation arrangements under which the non-employee directors of Myriad Genetics, Inc. (the “Company,” “our” or “we”) are compensated for their service as directors of the Company, including as members of the various committees of our Board of Directors (the “Board”).
Annual Retainer (all members) | $60,000 | ||||
| Chair of the Board | $120,000 additional retainer | ||||
| Committee Chair Compensation | |||||
| Audit and Finance Committee | $28,000 additional retainer | ||||
| Compensation and Human Capital Committee | $20,000 additional retainer | ||||
| Nominating and Governance Committee | $15,000 additional retainer | ||||
| Research and Product Innovation Committee | $28,000 additional retainer | ||||
Committee Member Compensation (1) | |||||
| Audit and Finance Committee | $13,500 additional retainer | ||||
| Compensation and Human Capital Committee | $10,000 additional retainer | ||||
| Nominating and Governance Committee | $7,500 additional retainer | ||||
| Research and Product Innovation Committee | $13,500 additional retainer | ||||
(1) Other than each Committee Chair
Attendance
Non-employee directors do not receive any fees (other than the retainers outlined above) for attending Board or committee meetings. However, directors are reimbursed for any out-of-pocket costs and expenses incurred in attending Board and committee meetings.
Equity Awards
Under our 2017 Employee, Director and Consultant Equity Incentive Plan (as amended, the “2017 Plan”), non-employee directors may receive an award of equity in the Company. As recommended and determined by our Compensation and Human Capital Committee, and approved by our Board, on the date of each annual meeting of stockholders, we may grant to each non-employee director equity awards under the 2017 Plan. In addition, depending on the proximity to our annual meeting of stockholders, we may grant equity awards under the 2017 Plan to each new non-employee director upon his or her initial appointment to the Board; provided, however, that it is our policy that directors should generally not receive more than one equity award per year. The number of shares of restricted stock, restricted stock units and/or other equity awards granted will be determined by dividing $350,000 by the NASDAQ closing
trading price of our common stock on the date of the applicable annual meeting of stockholders or the date that such new non-employee director is appointed to the Board, as applicable. Restricted stock, restricted stock units and other equity awards granted to our non-employee directors may vest, in the discretion of the Board and/or Compensation and Human Capital Committee, (1) in the case of awards granted on the date of our annual meeting of stockholders, upon the earlier of (i) one year of service on the Board following the date of grant or (ii) the date of the next annual meeting of stockholders following such grant and (2) in the case of awards granted on the date that a new non-employee director is appointed to the Board, on the date that is one year following the date of such grant.
Options granted to our non-employee directors under the 2010 Employee, Director and Consultant Equity Incentive Plan (as amended, the “2010 Plan”) are exercisable after the termination of the director’s service on the Board for the remaining term of the applicable option to the extent such option was exercisable on the date of such termination. All options or restricted stock units granted to our non-employee directors will become fully exercisable or vested upon a change of control of Myriad or upon their death as provided for under the forms of award agreement for directors under our 2010 Plan or 2017 Plan, as applicable.
Exhibit 23.1
Consent of Independent Registered Public Accounting Firm
We consent to the incorporation by reference in the following Registration Statements of Myriad Genetics, Inc.:
1.Registration Statement on Form S-8 (File No. 333-245718) pertaining to the Myriad Genetics, Inc. Non-Qualified Stock Option Agreement, Performance-Based Non-Qualified Stock Option Agreement, Restricted Stock Unit Agreement, and Performance-Based Restricted Stock Unit Agreement,
2.Registration Statement on Form S-3 (File No. 333-226492) pertaining to the Myriad Genetics, Inc. shelf registration for the sale of common stock,
3.Registration Statement on Form S-8 (File No. 333-222913, File No. 333-229574, File No. 333-236324) pertaining to the Myriad Genetics, Inc. 2017 Employee, Director and Consultant Equity Incentive Plan,
4.Registration Statement on Form S-8 (File No. 333-185325) pertaining to the Myriad Genetics, Inc. 2012 Employee Stock Purchase Plan,
5.Registration Statements on Form S-8 (File No.’s 333-171994, 333-179281, 333-185325, 333-193767, 333-209354 and 333-215959) pertaining to the Myriad Genetics, Inc. 2010 Employee, Director and Consultant Equity Incentive Plan, as amended, and
6.Registration Statements on Form S-8 (File No.’s 333-115409, 333-120398, 333-131653, 333-140830, 333-150792, 333-157130 and 333-164670) pertaining to the Myriad Genetics, Inc. 2003 Employee, Director and Consultant Stock Option Plan, as amended;
of our reports dated March 16, 2021, with respect to the consolidated financial statements of Myriad Genetics, Inc. and the effectiveness of internal control over financial reporting of Myriad Genetics, Inc. included in this Transition Report (Form 10-K) of Myriad Genetics, Inc. for the six-month period ended December 31, 2020.
/s/ Ernst & Young LLP
Salt Lake City, UT
March 16, 2021
Exhibit 31.1
SARBANES-OXLEY SECTION 302 CERTIFICATION
I, Paul J. Diaz, certify that:
1.I have reviewed this transition report on Form 10-K of Myriad Genetics, Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a)designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
5.The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a)all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b)any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
| Date: March 16, 2021 | |||||
| /s/ Paul J. Diaz | |||||
| Paul J. Diaz | |||||
| President and Chief Executive Officer | |||||
Exhibit 31.2
SARBANES-OXLEY SECTION 302 CERTIFICATION
I, R. Bryan Riggsbee, certify that:
1.I have reviewed this transition report on Form 10-K of Myriad Genetics, Inc.;
2.Based on my knowledge, this report does not contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements made, in light of the circumstances under which such statements were made, not misleading with respect to the period covered by this report;
3.Based on my knowledge, the financial statements, and other financial information included in this report, fairly present in all material respects the financial condition, results of operations and cash flows of the registrant as of, and for, the periods presented in this report;
4.The registrant’s other certifying officer(s) and I are responsible for establishing and maintaining disclosure controls and procedures (as defined in Exchange Act Rules 13a-15(e) and 15d-15(e)) and internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) for the registrant and have:
a)designed such disclosure controls and procedures, or caused such disclosure controls and procedures to be designed under our supervision, to ensure that material information relating to the registrant, including its consolidated subsidiaries, is made known to us by others within those entities, particularly during the period in which this report is being prepared;
b)designed such internal control over financial reporting, or caused such internal control over financial reporting to be designed under our supervision, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles;
c)evaluated the effectiveness of the registrant’s disclosure controls and procedures and presented in this report our conclusions about the effectiveness of the disclosure controls and procedures, as of the end of the period covered by this report based on such evaluation; and
d)disclosed in this report any change in the registrant's internal control over financial reporting that occurred during the registrant's most recent fiscal quarter (the registrant's fourth fiscal quarter in the case of an annual report) that has materially affected, or is reasonably likely to materially affect, the registrant's internal control over financial reporting; and
5.The registrant’s other certifying officer(s) and I have disclosed, based on our most recent evaluation of internal control over financial reporting, to the registrant’s auditors and the audit committee of the registrant’s board of directors (or persons performing the equivalent functions):
a)all significant deficiencies and material weaknesses in the design or operation of internal control over financial reporting which are reasonably likely to adversely affect the registrant’s ability to record, process, summarize and report financial information; and
b)any fraud, whether or not material, that involves management or other employees who have a significant role in the registrant’s internal control over financial reporting.
| Date: March 16, 2021 | |||||
| /s/ R. Bryan Riggsbee | |||||
| R. Bryan Riggsbee | |||||
| Chief Financial Officer | |||||
Exhibit 32
Certification
Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
(Subsections (a) and (b) of Section 1350, Chapter 63 of Title 18, United States Code)
Pursuant to section 906 of the Sarbanes-Oxley Act of 2002 (subsections (a) and (b) of section 1350, chapter 63 of title 18, United States Code), each of the undersigned officers of Myriad Genetics, Inc., a Delaware corporation (the “Company”), does hereby certify, to such officer’s knowledge, that:
The Transition Report on Form 10-K for the transition period ended December 31, 2020 (the “Form 10-K”) of the Company fully complies with the requirements of Section 13(a) or 15(d) of the Securities Exchange Act of 1934, and the information contained in the Form 10-K fairly presents, in all material respects, the financial condition and results of operations of the Company.
| Date: March 16, 2021 | Date: March 16, 2021 | |||||||
| /s/ Paul J. Diaz | /s/ R. Bryan Riggsbee | |||||||
| Paul J. Diaz | R. Bryan Riggsbee | |||||||
| President and Chief Executive Officer | Chief Financial Officer | |||||||
